
July 13, 2009 – Daiichi Sankyo Inc. and Eli Lilly and Company said Friday the FDA approved Effient (prasugrel) tablets for the reduction of thrombotic cardiovascular events (including stent thrombosis) in patients with acute coronary syndromes who are managed with percutaneous coronary intervention (PCI).
Effient helps keep blood platelets from sticking together to form clots, which can block an artery. The companies said taking Effient with aspirin after PCI has been shown to reduce the chances of having a cardiac event and stent-related blood clots among patients with acute coronary syndromes (ACS).
Effient should be initiated with a loading dose of 60 mg followed by a maintenance dose of 10 mg once daily, the companies said. In addition, for those patients who weigh less than 132 pounds (60 kg), physicians should consider lowering the maintenance dose to 5 mg once daily. Patients taking Effient should also take 75 mg to 325 mg aspirin orally once daily, according to their doctors’ instructions.
“The data from the TRITON-TIMI 38 Phase III pivotal trial provide compelling evidence that treatment with prasugrel significantly reduced the combined risk of cardiovascular death, heart attack or stroke over the current standard of care, clopidogrel, across a wide variety of patient types,” said lead TRITON-TIMI 38 investigator Elliott Antman, M.D., professor of Medicine at Brigham and Women’s Hospital (BWH) in Boston and senior investigator with the BWH TIMI Study Group. “Prasugrel is an important new option for patients with ACS who are managed with PCI. Prasugrel was associated with a significantly higher risk of serious bleeding events compared with clopidogrel. However, appropriate patient selection may help reduce this risk.”
The risk of bleeding was highest in Effient-treated patients who were either age 75 or older, weighed less than 132 pounds (60 kg), or who had a prior history of transient ischemic attacks (TIA) or stroke. Effient is contraindicated in patients with a history of prior TIA/stroke. It is generally not recommended in patients 75 years of age or older, except for patients in high-risk situations, such as those with diabetes or a history of prior heart attack.
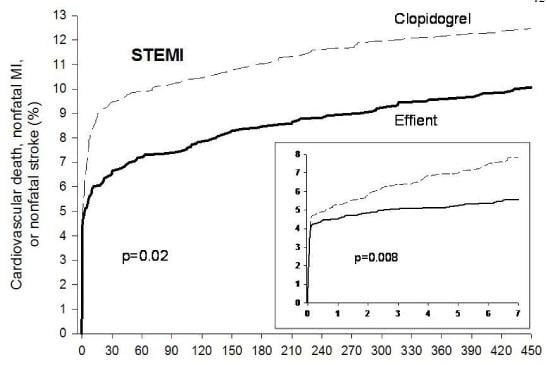
The approval of the drug was based on results from the pivotal phase III TRITON-TIMI 38 clinical trial, which compared Effient with Plavix (clopidogrel bisulfate) in reducing cardiovascular events in 13,608 acute coronary patients managed with PCI. The study showed that Effient taken with aspirin had a 19 percent relative risk reduction of the combined endpoint of cardiovascular death, nonfatal heart attack or nonfatal stroke versus Plavix taken with aspirin. This benefit was driven predominantly by reduction in heart attacks, the companies said. The benefit of Effient compared with Plavix was seen as early as three days and continued over the 15 months of the trial. In addition, there were fewer stent-related clots in patients treated with Effient compared with Plavix (a relative risk reduction of about 50 percent).
The risk of noncoronary artery bypass graft (nonCABG) related bleeding, which included life-threatening and fatal bleeding, was significantly higher with Effient (2.2 percent) compared with Plavix (1.7 percent). When compared with the overall treatment population, the risk of major bleeding was highest among those patients treated with Effient who were either 75 years or older, had a body weight less than 132 pounds, or had a prior history of transient ischemic attack/stroke.
An analysis from TRITON-TIMI 38, weighing the risk of major bleeding and the reduction in cardiovascular events, found an overall benefit significantly favoring Effient compared with Plavix. For every 1,000 patients treated with Effient as compared with Plavix, there were 23 fewer patients with heart attacks and six more with major bleeding events.
For more information: www.Effient.com, www.dsi.com


 January 28, 2026
January 28, 2026 









