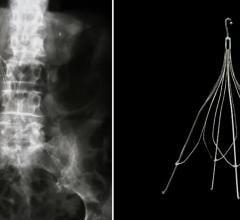
July 20, 2012 — Crux Biomedical announced it has received U.S. Food and Drug Administration (FDA) clearance for its novel inferior vena cava filter (VCF) with bi-directional retrieval. VCF are designed to trap blood clots that can lead to potentially fatal pulmonary embolisms among patients at risk. The Crux VCF is the first and only VCF designed to facilitate bi-directional retrieval through either the femoral or jugular veins, a key consideration when access to one or the other vein is limited. The device’s novel helical shape was designed to self-center and to conform more closely to the shape of the vena cava, as well as to reduce bends and stress that can compromise filter integrity.
To see a video of how the Crux Vena Cava Filter (VCF) operates, go to www.dicardiology.net/view-all/videos/?bclid=922766709001&bctid=1751927219001
"We have been pleased with both the clinical outcomes in our pivotal clinical trial and the enthusiasm expressed by physicians using the Crux VCF,” said Mel Schatz, CEO of Crux Biomedical.
Each year in the United States, approximately 600,000 patients develop pulmonary embolisms, and an estimated 200,000 deaths occur. The primary means of prevention and therapy for pulmonary embolisms is systemic administration of anticoagulant agents. However, this treatment is contraindicated in many patients, including those with high bleeding risk, those undergoing complex surgeries and patients for whom anticoagulants were ineffective.
Patients in whom anticoagulant treatment is contraindicated require alternate treatments to reduce the ongoing pulmonary embolism risk, including VCF. The FDA has recommended that physicians routinely remove VCF after the risk of pulmonary embolisms is reduced to avoid long-term complications.
"Crux designed a device that is both more versatile and simple to use," said Tom Fogarty, M.D., founder of Crux Biomedical. “Bi-directional deployment and retrieval are extremely helpful in situations where access to either the femoral or jugular vein is not possible. The Crux VCF, with its innovative design and materials, represents a paradigm shift in prevention of pulmonary embolisms in patients at risk.”
A recently completed pivotal trial consisting of 125 patients at high risk for pulmonary embolisms, called RETRIEVE, was performed at 22 sites in the U.S., Australia, New Zealand and Belgium. The study results were presented at the 2012 Society for Interventional Radiology (SIR) meeting.
In the study, the technical success rate of filter deployment was 98 percent; filter retrieval success was also 98 percent. The average retrieval time was 7 minutes. By the six-month follow-up, no embolizations, migrations or fractures were observed.
“The Crux device demonstrated excellent deployment, retrieval and safety profile,” said Robert R. Mendes, M.D., principal investigator of the study. “The clinical study evaluation has demonstrated the Crux VCF can be used safely for the prevention of recurrentpulmonary embolisms.”
For more information: www.cruxbiomedical.com


 July 27, 2021
July 27, 2021