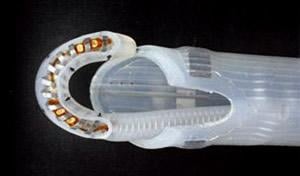
January 18, 2013 — nContact Inc. announced that the U.S. Food and Drug Administration (FDA) has cleared the Company’s modifications to its VisiTrax cardiac ablation device, EPi-Sense, now with embedded sensing capability. This next generation of technology includes sensors along the ablation device, which provide real-time feedback to physicians conducting cardiac ablation procedures.
The EPi-Sense device and its sensing technology allows electrophysiologists (EPs) to follow epicardial device positioning and lesion creation in real time by utilizing navigational mapping. From a safety perspective, the sensors use electrical feedback to ensure the device is properly positioned on cardiac tissue and energy is directed into the heart. Additionally, by using the sensors during epicardial ablation, the ablated tissue’s electrical impulses can be measured to predict lesion completeness before repositioning the device.
“The EPi-Sense is another step forward in advancing epicardial ablation techniques to address limitations of other ablation devices, potentially creating a better experience for physicians, and ultimately, patients,” commented John Funkhouser, president of nContact Inc.
“Throughout the company’s short history, their engineers have consistently innovated and improved devices for epicardial ablation, especially for the treatment of arrhythmias in patients with enlarged and diseased atria. This has allowed physicians and surgeons to work together to address and treat arrhythmias in patients with the most complex heart disease,” said Paul Mounsey, M.D., professor and Director of Electrophysiology at the University of North Carolina-Chapel Hill.
nContact will have the EPi-Sense device available for review at the Boston Atrial Fibrilliation Symposium Jan. 17-19, 2013, in Boston, Mass., at booth number 501. Commercial release of the device is scheduled for early 2013.
For more information: www.ncontactinc.com.


 February 06, 2026
February 06, 2026 









