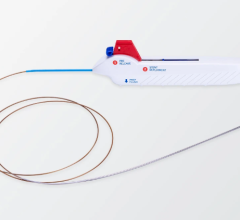
Concentric Medical has obtained FDA 510(k) clearance for its Merci L6 Retriever, a device that restores blood flow to ischemic stroke patients. The Merci Retriever is designed to restore blood flow by engaging, capturing and removing blood clots. It is a shaped wire constructed of nitinol, a memory wire, which allows delivery of the Retriever in linear form using standard catheterization techniques. A small puncture in the groin is used to introduce the Merci Retriever into an artery leading to the brain. Upon reaching the targeted area, the Retriever returns to its original shape when deployed. The Merci L6 Retriever joins the L family of retrievers, incorporating a 2.7mm cylindrical helix with attached filaments. The filaments provide an additional mechanism for securing the clot during retrieval.
If you enjoy this content, please share it with a colleague
FDA Clears Neurovascular Retriever for Use in Stroke Patients
Related Content
Feb. 2, 2026 — Brainomix, a provider of AI-powered imaging tools in stroke and lung fibrosis, is introducing its new ...
Jan. 13, 2026 — A streamlined, nurse-led response for hospitalized patients experiencing an acute stroke at a Texas ...
Dec. 3, 2025 — Recross Cardio Inc., a structural heart company developing next-generation membrane sealing technology ...
Dec. 2, 2025 — Brainomix, a provider of AI-powered imaging tools in stroke and lung fibrosis, has announced the ...
Nov. 10, 2025 — Sentante, a med-tech robotics company, has successfully demonstrated a first-of-a-kind remote stroke ...
Oct. 22, 2025 — Basking Biosciences, a clinical-stage biopharmaceutical company developing the first reversible ...
Oct. 21, 2025 — At the seventeenth edition of the World Stroke Congress in Barcelona, Siemens Healthineers will present ...
Aug. 19, 2025 — Openwater, an open-source medical technology company delivering portable, hospital-grade diagnostic and ...
Aug. 19, 2025 — In a move to advance stroke care and accelerate critical decision-making, Tift Regional Medical Center ...
July 9, 2025 — InspireMD, Inc., developer of the CGuard Prime carotid stent system for the prevention of stroke ...


 February 02, 2026
February 02, 2026