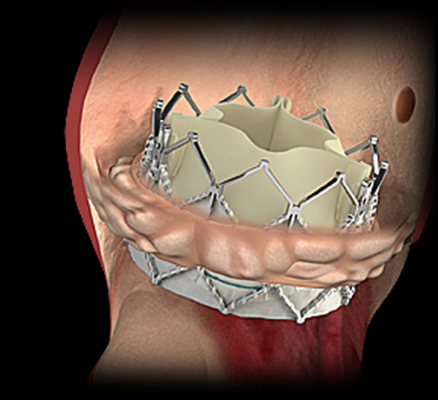
October 15, 2015 — U.S. Food and Drug Administration (FDA) has cleared Edwards Lifesciences Sapien XT transcatheter aortic valve for aortic valve-in-valve (VIV) procedures. It became the second transcatheter valve to receive the VIV indication. The Medtronic CoreValve received the indication this past March.
"U.S. approval of the valve-in-valve procedure provides an important minimally invasive treatment option for patients who are at high risk for a subsequent open-heart surgery to replace their bioprosthetic valves," said Larry L. Wood, Edwards' corporate vice president, transcatheter heart valves.
The news of the new FDA indication came today at the 27th Transcatheter Cardiovascular Therapeutics (TCT), the annual scientific symposium of the Cardiovascular Research Foundation, in San Francisco, one-year patient outcomes were presented for patients treated with transcatheter Sapien XT valve implantation in surgical tissue valves. The valve-in-valve procedure was associated with a high overall one-year survival rate of 86.6 percent and a low overall stroke rate of 3.7 percent in a very high-risk patient population, according to independently adjudicated data from the 197-patient PARTNER II Valve-in-Valve study. The study, which is a multicenter, non-randomized cohort of The PARTNER II Trial, included 97 patients enrolled in the primary registry as well as 100 continued access patients.
"We were very pleased to see 100 percent survival at 30 days with the 100 high-risk patients treated with the Sapien XT valve-in-valve procedure in the continued access registry," said Danny Dvir, M.D., interventional cardiologist at the Center for Heart Valve Innovation at St. Paul's Hospital, Vancouver, who presented the data. "This is quite remarkable and supports transcatheter aortic valve-in-valve replacement with the Sapien XT valve as a safe therapeutic alternative to re-operation for patients in need of a subsequent tissue valve replacement."
The Edwards Sapien XT valve was approved by the FDA in June 2014 for patients at high risk for native aortic valve replacement surgery, and received CE mark for valve-in-valve procedures in early 2014. The Sapien valve platform has been used in the treatment of more than 100,000 patients worldwide.
For more information: www.edwards.com


 December 24, 2025
December 24, 2025 









