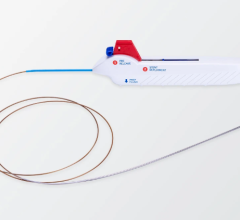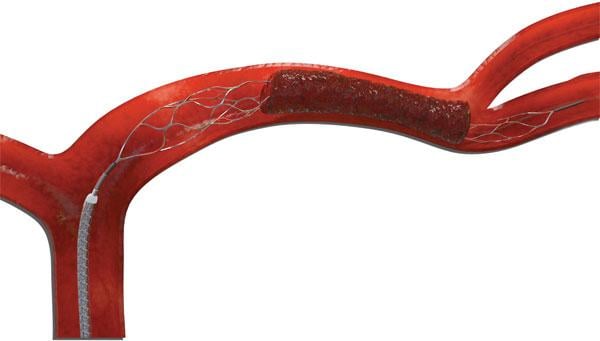

August 14, 2012 — The U.S. Food and Drug Administration (FDA) has granted 510(k) market clearance to Stryker Neurovascular’s Trevo Pro Retriever, indicated for the removal of blood clots. It uses Stentriever technology to optimized clot integration and retrieval in patients experiencing acute ischemic stroke.
The device demonstrated strong clinical results in the TREVO 2 clinical trial presented at the 2012 European Stroke Conference in May. This technology demonstrated the highest rate of revascularization in a randomized embolectomy stroke device trial, and achieved significantly better post-procedure revascularization than the Merci Retriever (92 percent in the Trevo Retriever arm compared to 76.7 percent in the Merci Retriever arm). Other measures of performance also strongly favored the Trevo Pro Retriever, including improvement in the National Institutes of Health Stroke Scale (NIHSS) score, excellent composite safety endpoints and shorter hospital stays.
"The launch of this device is another great stride in the evolution of stroke care," said TREVO 2 investigator Gary Duckwiler, M.D., University of California Los Angeles Medical Center. "Concentric Medical, which is now owned by Stryker, has a long history of partnering with physicians to develop devices specifically designed to remove blood clots from a blocked artery in the brain. The new Trevo Pro Retriever is easy to use and very effective at opening blood vessels; allowing physicians to have a significant impact on clinical outcomes."
"In this patient population, rapidly restoring blood flow to the brain is critical," said neurologist Wade Smith, M.D., University of California San Francisco Medical Center. "This technology advances our ability to help many patients avoid the devastating effects from a large stroke if they can get to a comprehensive stroke center quickly for treatment."
For more information: www.stryker.com


 February 02, 2026
February 02, 2026