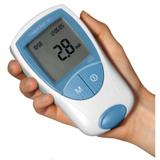
March 31. 2010 – The FDA granted Clinical Laboratory Improvement Amendments (CLIA)-waived status to expand the use of a point-of-care anti-coagulation monitor to a broader range of clinical settings. The CoaguChek XS Plus system offers connectivity and data management tools to help healthcare professionals manage PT/INR testing.
The waiver means labs that do not meet the requirements to do moderate- or high-complexity testing can now use the device. The waiver allows healthcare professionals in CLIA-waived environments to have access to new tools and connectivity options to help them manage patients on warfarin therapy. The technology has several features for fast, accurate results, and the connectivity capabilities help healthcare providers with regulatory compliance.
The CoaguChek XS Plus system works with the RALS-Plus information management system, which provides reporting and device management capabilities, to help hospital staff streamline the regulatory compliance process, capture reimbursable costs, and improve their organizational efficiency. In addition, recent enhancements to the system include the ability to hold up to 1,000 patient results and the reduction of the sample size requirement to 8 microliters.
The system uses two-level, built-in quality controls to help ensure the accuracy of PT/INR test results, but also offers optional liquid quality controls for facilities with policies requiring the use of external quality control measures.
For more information: www.roche.com, www.roche-diagnostics.us


 October 09, 2019
October 09, 2019 









