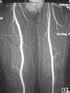
Ablavar has an MRA indication to evaluate aortoiliac occlusive disease.
January 20, 2010 - The first FDA-cleared contrast imaging agent for use with a magnetic resonance angiography (MRA) indication to evaluate aortoiliac occlusive disease (AIOD) and suspected peripheral vascular disease enters the U.S. market today. AIOD is a type of peripheral vascular disease that occurs when arteries, which carry blood from the heart to the lower limbs, become narrowed or blocked. This lack of blood supply can result in pain, infection and even loss of limbs[i]. In Phase 3 clinical studies, Ablavar, the contrast agent that Lantheus Medical Imaging launched today, demonstrated statistically greater sensitivity (detecting disease when present) compared with noncontrast MRA[ii]. These studies, which supported the U.S. Food and Drug Administration (FDA) approval of Ablavar, show that MRA images using Ablavar provided diagnostic accuracy comparable to conventional X-ray angiography,[iii],[iv] an invasive procedure which involves insertion of a catheter into the arteries in the upper thigh (groin area) or arm[v]. “Ablavar provides distinct advantages over X-ray angiography, the current standard of care in diagnosing AIOD,” said Mark G. Hibberd, M.D., Ph.D., senior medical director, Global Medical Affairs, Lantheus Medical Imaging, Inc. “Ablavar provides high resolution images comparable to conventional X-ray angiography (the current gold standard), which offers radiologists a clear, enhanced visualization of patients’ arteries. However, Ablavar is given in a single, low dose injection, does not require catheter insertion into a patient’s arteries, and does not expose patients to ionizing radiation, all of which are tangible benefits to patients.” According to E. Kent Yucel, M.D., FACR, chairman of radiology, Tufts Medical Center, the new contrast agent "may provide clinicians performing vascular imaging with more comprehensive, three-dimensional diagnostic information to improve patient treatment decisions and care." The introduction of the MRA imaging agent extends Lantheus Medical's product portfolio in the radiology and peripheral vascular disease markets and adds to its diagnostic imaging agents for MRI, SPECT, PET and echocardiography imaging modalities. Ablavar is approved in 37 countries outside the United States and has been used in nearly 90,000 patients to date. Contraindications: History of a prior allergic reaction to a gadolinium-based contrast agent. Gadolinium-based contrast agents increase the risk of nephrogenic systemic fibrosis (NSF) in patients with: - acute or chronic severe renal insufficiency (glomerular filtration rate <30 mL/min/1.73m2), or acute renal insufficiency of any severity due to the hepato-renal syndrome or in the perioperative liver transplantation period. In these patients, avoid use of gadolinium-based contrast agents unless the diagnostic information is essential and not available with noncontrast enhanced magnetic resonance imaging (MRI). NSF may result in fatal or debilitating systemic fibrosis affecting the skin, muscle, and internal organs. Screen all patients for renal dysfunction by obtaining a history and/or laboratory tests. When administering a gadolinium-based contrast agent, do not exceed the recommended dose and allow a sufficient period of time for elimination of the agent from the body prior to any re-administration. References: [i] Vascular Web. Society for Vascular Surgery. "Aortoiliac Occulsive Disease." http://www.vascularweb.org/patients/NorthPoint/Aortoiliac_Occlusive_Disease.html. Assessed Oct. 26, 2009. [ii] Data on file, Lantheus Medical Imaging Inc. [iii] Goyen M, Edelman M, Perreault P, et al. "MR Angiography of Aortoiliac Occulsive Disease: A Phase III Study of the Safety and Effectiveness of the Blood-Pool Contrast Agent MS-325." Radiology. 2005; 236(3):825-833. [iv] Rapp JH, Wolff SD, Quinn SF, et al. "Aortoiliac Occulsive Disease in Patients with Known or Suspected Peripheral Vascular Disease: Safety and Efficacy of Gadofosveset-Enhanced MR Angiography – Multicenter Comparative Phase III Study." Radiology. 2005; 236(1):71-78. [v] American Heart Association. "What is Coronary Angiography?" http://www.americanheart.org/presenter.jhtml?identifier=11222. Assessed on Oct. 26, 2009. [vi] The Imaging Market Guide (U.S. Edition 2009), Arlington Medical Resources Inc. Malvern, Pa.. [vii] National Heart, Lung, and Blood Institute. National Institutes of Health. "Other Names for Peripheral Arterial Disease." http://www.nhlbi.nih.gov/health/dci/Diseases/pad/pad_onames.html. Assessed March 30, 2009. [viii] National Heart, Lung, and Blood Institute. National Institutes of Health. "Who Is At Risk for Peripheral Arterial Disease?" http://www.nhlbi.nih.gov/health/dci/Diseases/pad/pad_risk.html. Assessed March 30, 2009. For more information: www.ablavar.com and www.lantheus.com


 August 17, 2023
August 17, 2023 








