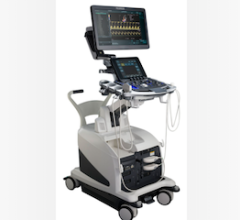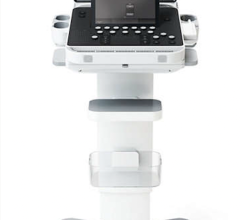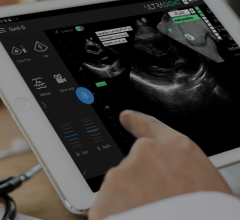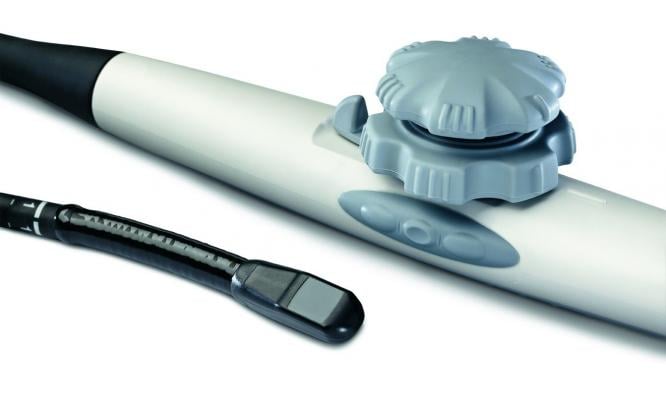
April 27, 2012 — GE Healthcare announced the U.S. Food and Drug Administration (FDA) clearance and availability of the latest version of its Vivid E9 cardiovascular ultrasound system. The Vivid E9 Breakthrough 2012 (BT12) includes a 4-D transducer for transesophageal echocardiography (TEE) and also provides innovative tools designed to help improve workflow efficiency through simplified image acquisition, intuitive navigation and advanced, yet easy to use, quantification.
While Vivid E9 is currently used most frequently in the echo lab, the availability of the 4-D TEE transducer enables the system to be used more often in settings including the cath lab and the operating room. The TEE transducer will allow clinicians to view precise images of the heart during assessment/diagnosis performed in the echo lab and to support invasive surgical procedures in the operating room, as well as image-guided procedures in the cath lab. With the TEE transducer, Vivid E9 can now be leveraged for procedures including mitral valve repair, transcatheter aortic valve repair (TAVR/TAVI), atrial septal defect (ASD) closures and patent foraman ovale (PFO) closures.
“With a recent increase in TEE procedures, our customers in the cath lab, echo lab and operating room have been looking for ways to achieve the benefits of 4-D imaging without compromising productivity,” said Al Lojewski, general manager of cardiovascular ultrasound at GE Healthcare. “Our Vivid E9 system was designed to help make 4-D imaging easier and more efficient. With the new 4-D TEE transducer as well as several important workflow enhancements, this latest version of Vivid E9 underscores our commitment to making 4-D imaging accessible and efficient across the continuum of care in cardiology.”
“The new 4-D transesophageal probe developed by GE Healthcare is an advanced diagnostic tool,” said Luigi P. Badano of the University of Padua, Padua, Italy. “It combines ease-of-use and flexibility to acquire 4-D data sets, user-friendly navigation tools that allow users to quickly visualize the cardiac structure of interest and effective quantification software to quantitate left ventricular pump function, myocardial deformation and mitral valve morphology.”
The Vivid E9 BT12 also includes several features that are new to cardiovascular ultrasound and which are designed to enhance 4-D imaging and workflow. These include the industry’s first configurable TEE transducer, a new tilt and rotate function, and a live “2-Click Crop” tool. Workflow enhancements can also be achieved during mitral valve acquisition with a dedicated MV button and during quantification with a new plug-in tool that has the potential to decrease assessment time.
“Vivid E9 BT12 with the new 4-D TEE probe has worked great, especially during our intense first two days of MitraClipprocedures,” said Reidar Winter of the Karolinska University Hospital, Stockholm, Sweden. “The system has tools like Biplane Prepare, Biplane Tilt/Rotate and Laser Lines that help optimize guidance of the catheter septal puncture as well as the mitral clip positioning, which has the potential to reduce procedure time.”
Vivid E9 BT12 is also CE marked and available for sale across Europe and many countries around the world.
New features help improve image quality, quantification and workflow and include:
Simplified Image Acquisition
- Triplane imaging, 4-D views and high frame rates for both TTE and TEE procedures
- One-button acquisition of mitral valve images
- In biplane mode, the ability to tilt and rotate at the same time; e.g., to more accurately image the mitral valve during a MV clip procedure
- A new QuickRotate button provides consistency and accuracy by allowing users to rotate an image in 30 degree increments with a single click
- Enhanced zoom capabilities allow for high-volume rates in a single- and multibeat in 4-D and 4-D color zoom mode using FlexiZoom
Intuitive Navigation
- 2-click crop, a quick and intuitive way of obtaining any 4-D view in both live and replay modes
- Laser lines, a new tool to help understand the relationship between 2-D slices, and 4-D views, help to visualize the linkage between 2-D and 4-D, and also helps improve depth perception
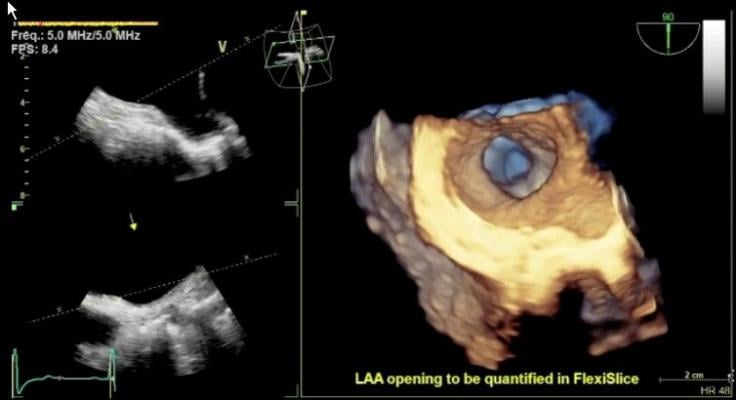
- New “one-stop-shop” slice tool, FlexiSlice, provides full flexibility for the user to slice in any direction, 2-D view or render view in both live and replay modes
Advanced Quantification
- A new MV Assessment plug-in from TomTec available on EchoPAC BT 12:
- Increases productivity during mitral valve assessment
- Provides a set of static and dynamic measurements describing the mitral valve anatomy.
- An automated left ventricular volume and EF quantification tool (4-D Auto LVQ) also for TEE
For more information: www.gehealthcare.com

 October 09, 2025
October 09, 2025