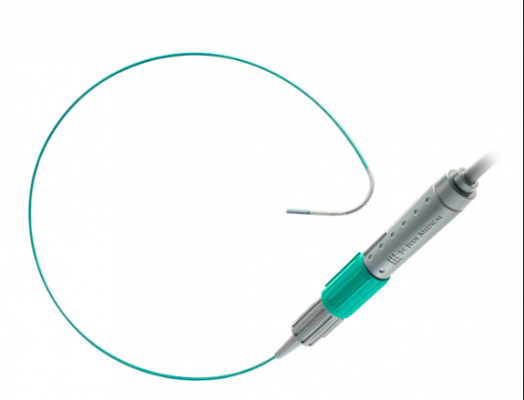
May 21, 2018 — Cardiology medical device reprocessing company Innovative Health recently received U.S. Food and Drug Administration (FDA) clearance to reprocess the Abbott ViewFlex Xtra Diagnostic Ultrasound Catheter. Ultimately, this clearance will allow the company to develop a full product suite for clinicians based on this new category of devices, including other widely used mapping and imaging catheters.
The company said it launched this product reprocessing effort based on direct feedback from its customers, including clinicians in the electrophysiology (EP) field.The device is much more complex to reprocess than many types of catheters, which required the FDA clearance.
Innovative Health continues to make extensive investments in research and development, enabling the company to pursue clearances for devices which are based on the very latest, difficult-to-reprocess technologies. In the last two years, the company has received three times more EP FDA clearances than all of its competitors combined. Reprocessors must actively pursue FDA clearances in order to introduce new products, which involves documenting that they can achieve levels of quality and safety comparable to those of the original device. This research can also help companies like Innovative Health identify entirely new reprocessing approaches, as it has with the ViewFlex catheter, allowing them to more easily pursue clearances for other new products based on similar technologies.
The ViewFlex catheter is used frequently in EP labs today to help visualize cardiac anatomy and physiology. With this recent FDA clearance, it will be added to Innovative Health’s vast portfolio of reprocessed devices include diagnostic, ultrasound and mapping catheters, as well as other EP-specific devices. Innovative Health partners with hospitals to collect the devices, then applies a rigorous, proprietary process to clean, test and sterilize them for re-use. As a result, the company claims it can help hospitals save hundreds of thousands of dollars on medical device spending per year, all while maintaining patient safety.
For more information: innovative-health.com


 January 29, 2026
January 29, 2026 









