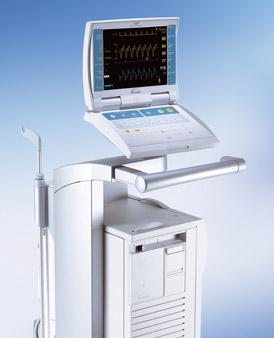
Maquet Datascope CS300 Intra-Aortic Balloon Pump
June 15, 2011 – The U.S. Food and Drug Administration (FDA) said Maquet Datascope Corp. has issued a class I recall for its System 98/98XT, CS100/CS100i and CS300 Intra-Aortic Balloon Pumps (IABPs) because of a defective fan in the power supply. The defect may cause overheating and shut down the device without visible or audible alarms. The company said consequences of unanticipated interruption of therapy could include the inability to decrease already-present ischemia, thrombus formation, organ injury or other serious events.
The recall includes 885 devices manufactured between May 2008 and December 2010. Affected lot/serial numbers include 7081436, 7081963, 7083463, and 7090616.
Affected models include:
- 0998-00-0446-xx
- 0998-UC-0446-xx
- 0998-00-0479-xx
- 0998-UC-0479-xx
- 0998-UC-0446Hxx
- 0998-UC-0479Hxx
- 0998-00-3013-xx
- 0998-UC-3013-xx
- 0998-00-3023-xx
- 0998-UC-3023-xx
IABPs are an electromechanical system used to inflate and deflate an intra-aortic balloon to provide temporary support to the left ventricle of the heart during cardiac surgery or interventional cath lab procedures.
Maquet Datascope notified customers by letter on March 17 describing the problem, the potential hazard, and the action to be taken. Customers were advised that their service representative would arrange to replace the power supply on affected devices, which would contain a new fan assembly.
Class 1 recalls are the most serious type of recall and involve situations in which there is a reasonable probability that use of these products will cause serious adverse health consequences or death.
The Maquet Technical Support Department can be reached at 800.777.4222 and press 4 (Monday through Friday 9 a.m. to 5:30 p.m. EST).
For more information: www.fda.gov/Safety/MedWatch/default.htm


 August 14, 2023
August 14, 2023 









