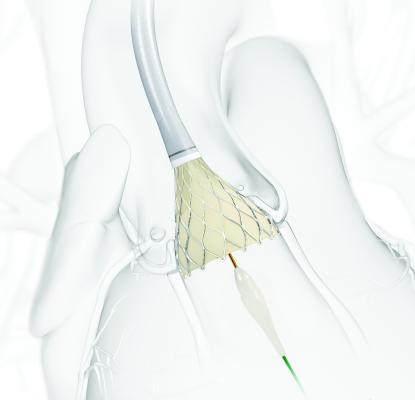
June 23, 2015 - Medtronic plc announced the U.S. Food and Drug Administration (FDA) approval and U.S. launch of the recapturable, self-expanding CoreValve Evolut R System. The first-and-only recapturable and repositionable device available in the United States, the Evolut R System is approved for transcatheter aortic valve replacement (TAVR) in severe aortic stenosis patients who are at high or extreme risk for surgery. Untreated, aortic valve stenosis can lead to serious heart problems including heart failure and even death.
The CoreValve Evolut R System is designed to treat patients with aortic stenosis - a condition where the aortic valve narrows, thereby limiting blood flow from the aorta to the rest of the body.
"In a short time, the TAVR procedure has become an established treatment option for high-risk patients with severe aortic stenosis who are unable to undergo surgery, and physicians are looking to technology advancements to help deliver even better patient outcomes," said Mathew Williams, M.D., co-primary investigator for the study, as well as chief of adult cardiac surgery and director of interventional cardiology and structural heart at the NYU Langone Medical Center in New York City. "Clinical data have shown the best patient outcomes are achieved when the valve is properly positioned. The advancement of recapturability with Evolut R gives physicians more confidence during the procedure and provides advantages that are non-existent in other TAVR systems."
The new system consists of the CoreValve Evolut R transcatheter valve and the EnVeo R Delivery System, which features an InLine Sheath that significantly reduces the profile to one of the lowest on the market (14 Fr equivalent, less than 1/5 inch). A smaller profile size provides a greater opportunity to treat an expanded patient population with smaller vessels (down to 5mm), through the preferred transfemoral access route, which may minimize the risk of major vascular complications in some patients.
Based on the knowledge gained through experience with the CoreValve System, the Evolut R is optimized to increase conformability and sealing at the annulus, while maintaining supra-annular valve positioning for improved blood flow and hemodynamic performance. An extended sealing skirt on the 26mm and 29mm valve sizes is intended to further promote valve sealing at the annulus.
In March, the CoreValve System was the first TAVR system to be approved in the U.S. for valve-in-valve (VIV) procedures in patients whose surgical aortic heart valves have failed. Also in March, two-year data from the High Risk Study of the CoreValve U.S. Pivotal Trial was presented at the American College of Cardiology annual scientific sessions (ACC.15), which showed superior survival benefit at two years for TAVR with the CoreValve System compared to patients who underwent surgical aortic valve replacement (SAVR).
The 23-, 26- and 29mm sizes of the CoreValve Evolut R transcatheter valve and the CoreValve EnVeo R Delivery Catheter System are available for use in the United States. The device is also available in Europe and other countries that recognize the CE (Conformité Européene) mark.
For more information: www.medtronic.com


 December 24, 2025
December 24, 2025 









