May 8, 2015 — Pie Medical Imaging announces its new release of 3mensio Structural Heart, dedicated to planning of structural heart interventions. This new release contains an optimized Mitral workflow and a new Septal Crossing workflow for planning of mitral valve procedures to determine the appropriate access route based on computed tomography (CT) images. These new innovations will be shown at the EuroPCR in Paris, May 19- 22.
More insight on the size and shape of the mitral valve is vital for pre-procedural planning of mitral valve replacement and repair procedures. The Mitral workflow provides advanced visualization and sizing of the mitral annulus and its surrounding structures like the aortic valve and coronary arteries. Also the possible impact on the left ventricular outflow tract (LVOT) clearance of placing a valve can be simulated.
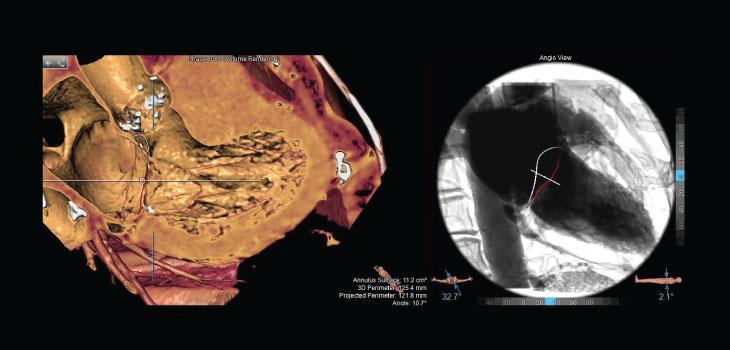
The Septal Crossing workflow enables visualization of cardiac structures in a simulated angioview. In this simulated angioview, anatomic relations between the interatrial septum, left atrial appendage (LAA) ostium, mitral annulus and vena cava can be easily determined.
For more information: www.piemedicalimaging.com


 December 24, 2025
December 24, 2025 









