July 30, 2019 – Procyrion Inc. secured Breakthrough Device designation by the U.S. Food and Drug Administration (FDA) for its Aortix System. Aortix is a circulatory support device for chronic heart failure patients on medical management who have been hospitalized for acute decompensated heart failure (ADHF) with worsening renal function.
Breakthrough Designation is granted to certain medical devices and device-led combination products that provide a more effective treatment of life-threatening or irreversibly debilitating diseases. It enables manufacturers to provide patients and healthcare providers with timely access to medical devices by expediting their development, assessment and review. Benefits of this designation include frequent interactions and feedback from FDA during the premarket review phase. Through this program, Procyrion can expect prioritized review of its FDA submissions for the Aortix System. In addition, the Centers for Medicare and Medicaid Services (CMS) recently proposed a new rule1 that would provide coverage and increase payments for medical devices designated by the FDA as breakthrough devices, so Medicare beneficiaries do not have to wait for access to the latest innovations.
“With Aortix, we have a device innovation that truly warrants breakthrough designation for the treatment of ADHF patients with worsening renal function, a particularly challenging group of patients for whom outcomes are generally poor and treatment options limited,” commented Keith Aaronson, M.D., MS, Pitt Collegiate Professor of Cardiovascular Medicine and medical director of the Center for Circulatory Support at the University of Michigan.
Heart failure (HF) is common, affecting more than 6 million adults and resulting in more than 1 million annual hospitalizations in the United States alone.[2] HF costs the nation an estimated $30.7 billion each year.[3] HF is a chronic, progressive condition, marked by episodes of acute decompensation typically requiring hospitalization for stabilization and improvement in symptoms. Unfortunately, outcomes after hospitalization for decompensated heart failure are poor. The one-year mortality rate after a heart failure hospitalization has remained high at 20-30 percent,[4] and there is additive risk with each subsequent hospitalization.[5,6] In addition, at least moderate renal dysfunction (Stage III) is present in up to 64 percent of ADHF admissions,[7] and it is estimated that more than 325,000 admissions per year in the U.S. are for patients with ADHF who also have worsening renal function.[8] These patients represent a particularly high-risk population, as they are frequently non-responders to available medical therapy and have poor outcomes.
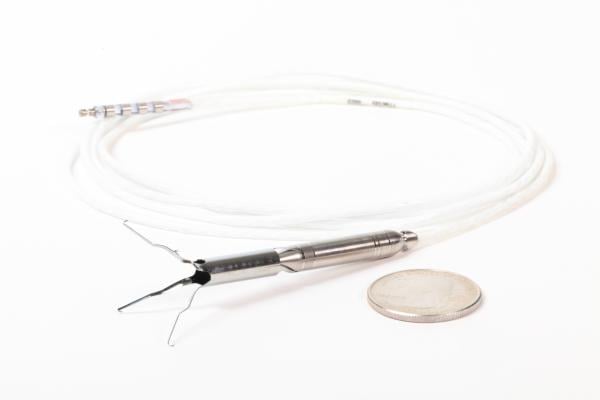
The Aortix System is a percutaneous circulatory support device for the treatment of heart failure. The initial version of the Aortix device provides up to seven days of circulatory support for chronic heart failure patients who have been hospitalized for ADHF, have worsening renal function, and are unresponsive to medical management. As demonstrated in animal studies[9] and a six-patient First-in-Human study,[10] Aortix benefits both the heart and the kidneys based on its unique design and placement in the descending thoracic aorta. Aortix can disrupt the harmful cardiorenal cycle in two ways: above the pump, it rests the heart by reducing aortic root pressure (afterload) resulting in increased cardiac output and decreased cardiac work; downstream, it provides increased blood flow to the kidneys resulting in increased urine output and a reduction in fluid overload. The device is placed via a minimally invasive catheter-based procedure that takes less than 10 minutes.
For more information: www.procyrion.com
Related Aortix Content
VIDEO: Advances in Interventional Heart Failure Hemodynamic Support
Aortix Heart Failure Pump Chosen for TCT “Shark Tank” Competition
Reducing Economic Burden of Cardiorenal Syndrome With The Aortix Pump
References:
1. https://s3.amazonaws.com/public-inspection.federalregister.gov/2019-08330.pdf


 February 03, 2026
February 03, 2026 









