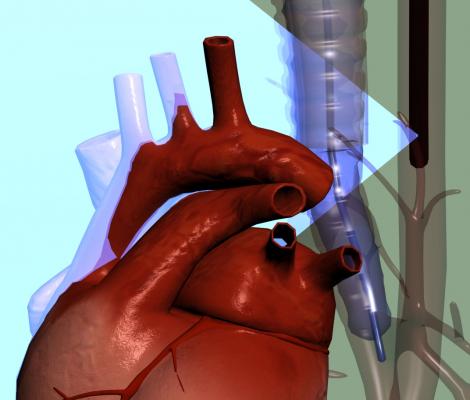

June 20, 2017 — Dutch medical device company Stroke2prevent BV recently announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for A-View, a medical device used in cardiac interventions. The A-View balloon catheter visualizes the aortic arch with transesophageal echocardiography (TEE).
A-View is intended to resolve the so-called blind spot of TEE, caused by interposition of the trachea. The device is used to provide imaging of the entire aortic arch and has demonstrated to detect atherosclerosis, diagnose aortic diseases and to monitor vascular flow. The additional information obtained by A-View supports surgeons to ensure the best patient outcomes.
A-View is currently marketed in Europe and will become available in the U.S. soon, pending arrangements for a proper distribution and clinical support channel.
For more information: www.stroke2prevent.com

 June 12, 2024
June 12, 2024