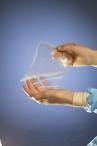
March 9, 2008 - Biomaterials company SyntheMed Inc. has received FDA approval of its pre-market approval (PMA) application for REPEL-CV Adhesion Barrier for use in pediatric cardiac surgery patients.
REPEL-CV is a bioresorbable adhesion barrier film based on SyntheMed’s proprietary polymer technology. When placed over the surface of the heart at the conclusion of an open-heart surgical procedure it is designed to reduce the severity of adhesions that form between the surface of the heart and adjacent tissue surfaces following the surgical procedure. REPEL-CV is designed to provide the therapeutic benefit and then degrade so that it is cleared from the surgical site.
In a randomized, controlled clinical trial conducted at 15 pediatric cardiac surgery centers throughout the U.S., over 70 percent of the REPEL-CV treated patients were completely free of clinically significant adhesions, the most severe grade of adhesions measured, as compared to less than 30 percent in the control patients.
For more information: www.synthemed.com


 February 13, 2026
February 13, 2026 









