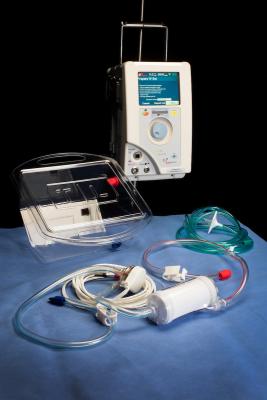
June 12, 2017 — TandemLife began its commercial launch of the TandemLife Priming Tray, a new product designed to simplify and speed up the process of putting critically-ill patients on extracorporeal life support, providing healthcare professionals with a vital window of opportunity to accurately assess a patient’s condition and provide appropriate care.
More than 500,000 Americans need emergent cardiac support each year, but fewer than 2 percent of these receive care with advanced extracorporeal life support (ECLS). Historically, ECLS has only been available for use within the operating rooms and intensive care units of large medical centers, due to the complexity of initiating this form of life support. TandemLife has the potential to change this paradigm by combining its TandemHeart pump and TandemLung oxygenator into a single, pre-connected packaging solution that also serves as a priming basin for the system. These medical devices are capable of assisting in the circulation and oxygenation of blood for a patient with compromised cardiac or respiratory function, and with the new TandemLife Priming Tray they can be deployed in just a few minutes, rather than up to an hour with existing technology. As a result, TandemLife could bring a high level of circulatory support capability to hospitals nationwide, regardless of their location, size or current treatment algorithm.
The priming tray was designed to provide hospitals with a simple, repeatable priming process that can be implemented quickly wherever cardiopulmonary support is needed.
For more information: Tandemlife.com


 January 15, 2026
January 15, 2026 









