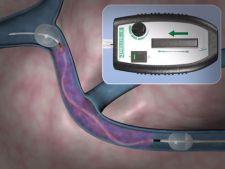
Bacchus Vascular Inc. has developed a catheter-based device, the Trellis-8 infusion catheter, which disperses thrombolytic solution directly into the clot of patients with deep vein thrombosis (DVT), is said to be safe and effective, according to data presented at the 33rd annual meeting of the Society of Interventional Radiology, held March 16, 2008.
The Trellis-8 infusion catheter is an alternatives to catheter-directed thrombolysis, which is sometimes used as an adjunct to anticoagulation in more severe DVT cases. The device is FDA approved for use in the peripheral vasculature and is more commonly used to treat peripheral arterial occlusions, and can reportedly help treat DVT.


 October 28, 2025
October 28, 2025 









