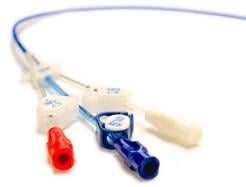
May 19, 2010 – Three distinct lumens in a new line of peripherally inserted central catheters (PICCs) increase flexibility to administer medications simultaneously. It also allows contrast power injections for computed tomography (CT) imaging using a single PICC line.
The Morpheus Smart Triple Lumen CT PICC was launched this week. It features a tapered catheter that quickly narrows from a 7 French diameter to 6 French. This improves blood flow and reduces the risk of thrombosis. The PICC’s catheter shaft technology couples the pushability needed for PICC placement with pliability that minimizes the risk of vessel trauma and phlebitis.
This is the first of several new catheters planned for the new Morpheus Smart PICC line.
For more information: www.angiodynamics.com


 October 28, 2025
October 28, 2025 









