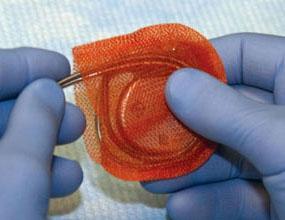
April 14, 2010 – An antibacterial wrap for implanted pacemakers and defibrillators released this week is designed to provide protection against infections. The AigisRx Flat delivers the antimicrobial agents rifampin and minocycline. "The current AigisRx envelope provides potent antimicrobial protection and also anchors the device to prevent device migration. However, in patients who are undergoing battery change-outs, using the AigisRx Flat option helps minimize dissection of the pre-existing pocket,” said Heather Bloom, M.D., assistant professor of medicine, Emory University School of Medicine, director, cardiac electrophysiology services, Atlanta Veterans Affairs Medical Center. “Since the risk of infection is much higher in change-outs, partially due to the vascular pocket preventing tissue penetration by oral antibiotics, having a drug-eluting device on the site is of critical importance." Hospitalization for device infection increased faster than rates of device implants from 1996 through 2006, according to the National Hospital Discharge Survey, conducted by the Centers for Disease Control and Prevention. This was further reinforced in the January 2010 guideline issued by the American Heart Association and the Heart Rhythm Society, which explicitly states there is a significant unmet clinical need in device-related infection. Following commercial release in 2008, Aigisrx envelope has been implanted in more than 8,500 patients nationwide. For more information: www.TYRX.com


 January 29, 2026
January 29, 2026 









