The FDA cleared Boston Scientific’s ALTRUA family of pacemakers, which enables the company to…
Thoratec Corp. received FDA approval of its PMA (premarket approval) application, allowing the…
The Sorin Group said the FDA approved to market the CRT model of its OVATIO family, reportedly…
Boston Scientific Corp. offers the PROMUS Everolimus-Eluting Coronary Stent System for the…
Biotronik GmbH launched the Lumax 540 series, which includes implantable cardioverter…
IntelliDOT Corp.
June 25, 2008 - Cardiologists completed the 1,300th worldwide procedure utilizing the…
June 25, 2008 - Lexiscan (regadenoson) injection, an A2A adenosine receptor agonist, for use as…
Medtronic has extended its line of CRT leads with Attain StarFix OTW (over-the-wire) lead (Model…
June 16, 2008 – Philips released today its new Philips BrightView XCT, reportedly the first time…
OrbusNeich's Scoreflex coronary dilatation catheter, which is only CE Mark approval, but not yet…
Acusphere has submitted a New Drug Application (NDA) to the FDA for approval to market Imagify (…
Card Guard AG has introduced its next-generation LifeStar ACT III Platinum system with 3-lead…
Braemar Inc. rolled out the DL900, a 1.75 ounce, compact digital Holter monitor with a large…
Spacelabs Healthcare recently introduced its newest clinical application suite, the ICS G2,…
The vProtect Luminal Shield is a self-expanding intracoronary prosthesis designed to limit…
Vascular Solutions is partnering with Radius Medical Technologies to distribute in the U.S. the…
Cook Medical's Zenith TX2 Thoracic TAA Endovascular Graft is designed for thoracic endovascular…
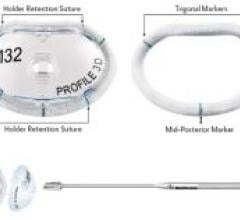
Medtronic introduced Profile 3D Annuloplasty Ring with a design based on the geometry of the…

 July 08, 2008
July 08, 2008