Nov. 12, 2025 — The Association of Black Cardiologists (ABC) has released new findings from a national survey exposing a ...
Nov. 13, 2025 — Esaote has announced a new strategic partnership with Schiller Americas. This collaboration strengthens ...
Nov. 12. 2025 — The American Heart Association’s Council on Clinical Cardiology has selected Roxana Mehran, MD, as the ...
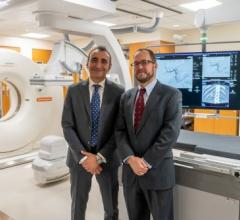
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
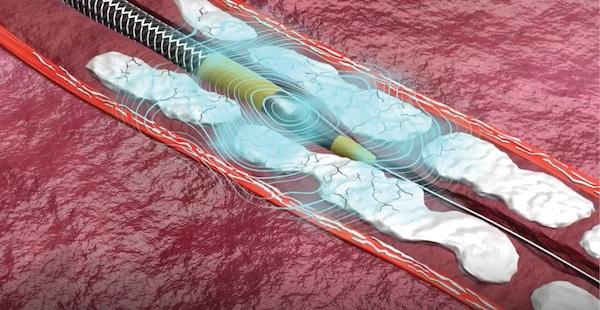
Since receiving FDA approval in 2016, intravascular lithotripsy (IVL) systems have grown in popularity among ...
Nov. 12, 2025 — Siemens has announced plans to deconsolidate its remaining stake in Siemens Healthineers (currently ...
Nov. 11, 2025 — FastWave Medical has successfully completed enrollment in its 30-patient coronary feasibility study and ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Nov. 12, 2025 — On Nov. 11, Huntsman Cancer Institute at the University of Utah (the U) opened its first specialized ...

Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Nov. 12, 2025 — AISAP, a provider of AI Point-of-Care Diagnostics, has announced a field initiative in Ghana, deploying ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Nov. 10, 2025 —Genomics, a science-led techbio company, has today announced new research that suggests polygenic risk ...
Nov. 10, 2025 — Kestra Medical Technologies, a wearable medical device and digital healthcare company, has announced ...
Nov. 10, 2025 — Stereotaxis has received U.S. Food and Drug Administration 510(k) clearance for its latest generation ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Nov. 8, 2025 — Sanjay Rajagopalan, MD, MBA, Chief of Cardiovascular Medicine at University Hospitals Harrington Heart & ...
Nov. 10, 2025 — Sentante, a med-tech robotics company, has successfully demonstrated a first-of-a-kind remote stroke ...
Nov. 4, 2025 — Amplitude Vascular Systems (AVS), a medical device company focused on treating calcified arterial disease ...


 November 17, 2025
November 17, 2025