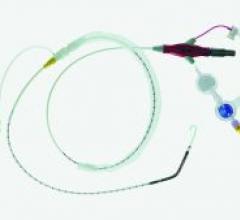
Vascular Insights received 510(k) clearance from the FDA to market its ClariVein infusion catheter for infusion of physician-specified agents in the peripheral vasculature. ClariVein is a percutaneous, 2 2/3 Fr (0.035-inch) catheter, containing a rotating wire driven by a motor, that enhances fluid dispersion in the treatment area.
July 9, 2008 – The Medical Imaging & Technology Alliance (MITA) today applauded the U.S. Congress for passing Medicare ...
The Abiomed Inc. Impella 2.5 cardiac assist device was FDA cleared in 2008 for use for partial circulatory support for periods up to six hours. The intra-aortic balloon pump (IABP) also has 510(k) clearance and approximately 110,000 are used each year in the U.S.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The FloTrac is reportedly the first minimally invasive hemodynamic monitoring device that connects to any existing arterial line and requires no manual calibration. The latest enhancement to the FloTrac software empowers clinicians with greater flexibility to trend and analyze the patient parameters in order to make better informed decisions, said the company.
July 9, 2008 - InnerSpace has added the V-Series line of heavy-duty cabinets, carts and workstations to its organized ...
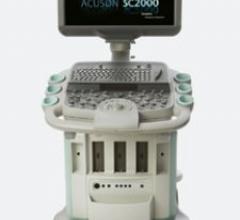
The ACUSON SC2000 volume imaging ultrasound system acquires nonstitched, real-time full-volume 3D images of the heart in ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
The Sorin Group said the FDA approved to market the CRT model of its OVATIO family, reportedly the smallest CRT defibrillator (CRT-D) available. The OVATIO CRT 6750 is shaped to offer ease of implantation and long-term patient comfort, and is designed to allow more flexibility in the management of cardiac resynchronization and antitachyarrhythmia therapy due to its Brady-Tachy Overlap (BTO).
Recently introduced at the Heart Rhythm Society (HRS 2008) meeting, the new DigiTrak XT Holter recorder is reportedly equipped with powerful and innovative technology aimed at simplifying the patient and clinical user experience.
July 9, 2008 - New research shows catheter cryoablation is comparably effective to radiofrequency catheter ablation for the treatment of atrial flutter (AFL) and may have some safety advantages over the use of radiofrequency.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Siemens' ACUSON SC2000 volume imaging ultrasound system acquires nonstitched, real-time full-volume 3D images of the heart in one single heart cycle. Called “Echo in a Heartbeat,” this new technology generates nonstitched full-volume imaging reportedly of the entire heart with 90 degree pyramids and in just one second.
Angiotech Pharmaceuticals Inc. received 510(k) clearance from the FDA to market its 5-Fluorouracil (5-FU) coated central venous catheter (CVC).
July 10, 2008 - The XIV Congress of the Latin American Society of Interventional Cardiology (SOLACI) and the annual ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The FDA cleared Boston Scientific’s ALTRUA family of pacemakers, which enables the company to introduce its first Boston Scientific-branded pacemaker to treat bradycardia.
Midmark Diagnostics Group’s (MDG) IQmark Advanced Holter is designed to help physicians improve the efficiency, workflow and quality of care. Its reportedly comprehensive functionality offers users the option to control the parameters of the analysis, edit results, select report functions and provides quick and easy access to the entire full disclosure.
July 9, 2008 – The American Heart Association recently issued new guidelines for computed tomography (CT) and magnetic ...


 July 08, 2008
July 08, 2008