Toshiba America Medical Systems Inc. announced the introduction of its Infinix VF-i/SP X-ray system, a universal cardiovascular system designed to accommodate diagnostic and interventional procedures. The system will be showcased at the American College of Cardiology (ACC) annual meeting in New Orleans March 24-27, 2007.
The Xper Information Management is Philips’ new personalized cardiovascular workflow solution. The product suite presents a variety of new innovations for reporting, image review, scheduling, inventory and intelligent data management. With tools that enhance efficiency on multiple levels, the solution improves and simplifies workflow for all cardiovascular professionals.
Agfa HealthCare's Scheduling for Cardiology solution, a Web-enabled scheduling solution for resources management within cardiovascular departments, has been designed to be configurable to multiple, personal-based workflows. Scheduling for Cardiology’s Web-based architecture provides viewing and management of resource availability from any point of access to the hospital network.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
In an effort to continue the successful launch of the s5i integrated IVUS system in the US and Europe, Volcano has ...
Siemens introduced the AXIOM Sensis XP - a new generation of integrated recording solutions that increases procedural efficiency, allowing for optimal patient care in the cath lab.
Siemens Medical Solutions previewed a hand-held ultrasound unit, the Acuson P10, intended primarily for triage and screening applications in acute care settings. In the future, personal imaging tools such as this could improve the standard of care for inpatient monitoring, critical care, outpatient specialty visits and bedside exams.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
ScImage announced that it is delivering a fully customizable reporting and workflow documentation solution for vascular procedures. The reporting solution will be offered as an add-on to the company’s award winning Enterprise PACS solution, PicomEnterprise.
The results of Boston Scientific's pivotal SPIRIT III clinical trial reaffirmed prior safety and efficacy data for the market-leading TAXUS Express2 Paclitaxel-Eluting Coronary Stent System and provided early positive data for the XIENCE V (PROMUS) Everolimus-Eluting Coronary Stent System.
As the pioneer of metabolic assessment and behavioral therapy technologies, BodyMedia is developing tools that empower health professionals to better treat patients with chronic health conditions such as obesity, diabetes and cardiovascular disease (CVD).
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Data released from the bridge-to-transplantation (BTT) arm of the HeartMate II Pivotal trial demonstrate that the device provides effective mechanical circulatory support as a bridge to transplantation for heart failure patients, and that these patients experience a lower number of adverse events and an improved quality of life while being supported by the device.
GE Healthcare announced the availability of the latest version of Centricity Cardiology CA1000 version 2.0, which provides unique tools for analysis, segmentation, measurements, annotation, filming and exporting of clinically relevant images.
The Virtual Explorer 3D Workstation is designed to analyze magnetic resonance (MR) images of the heart. It is a complete 3D, post-processing tool for MR analysis and display. The physician-friendly design offers a full range of features that will enhance MR imaging capability and functionality.
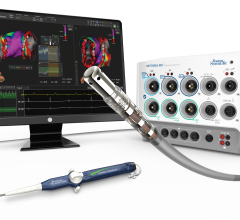
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Vital Images Inc. featured its next generation of ViTALCardia, which includes a new EP planning tool and CathView, a new angiographic orientation.
ZOLL Medical Corp. has received FDA clearance to market and sell the ZOLL M Series with Real CPR Help technology. The device enables rescuers to instantly see and hear how well they are performing the rate and depth of CPR chest compressions. The M Series is the Company’s best-selling product to date. The approval extends Real CPR Help to ZOLL’s complete defibrillator product line.
Boston Scientific Corp. announced two-year results from the TAXUS V In-Stent Restenosis (ISR) trial, demonstrating that the TAXUS Express2 paclitaxel-eluting coronary stent system met its primary endpoint in the treatment of in-stent restenosis - the regrowth of diseased tissue into a previously stented artery utilizing bare-metal stents - compared to vascular brachytherapy.

 March 27, 2007
March 27, 2007