In as much as the environment in which you live can affect your quality of life, so can the environment in which someone receives medical care affect patient outcome – at least in theory.
September 3, 2008 - CardioNet Inc. said today Humana upgraded the CardioNet System to a “covered benefit” status, from ...
The FDA has cleared Medtronic’s Attain StarFix OTW (over-the-wire) lead (Model 4195), an active fixation left-heart lead for cardiac resynchronization therapy (CRT).
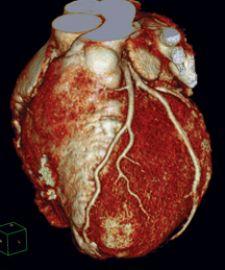
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The Xcelera R2.2 upgrade is a new version of the Xcelera multimodality cardiology image management, analysis and reporting solution, designed to offer enhanced functionality and greater workflow efficiency.
he Powerheart AED G3 is designed with Rescue Ready technology that self-checks all main components daily. The device ...

If the case went to court for whether positron emission tomography (PET) or single, photon emission computed tomography (SPECT) is better suited for myocardial perfusion imaging, experts say the current technology and developments on the horizon would result in a hung jury.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
September 3, 2008 – Research published this week in the New England Journal of Medicine said patients with heart failure in whom an ICD is implanted and receive shocks for any arrhythmia have a substantially higher risk of death than similar patients who do not receive such shocks.
Boston Scientific launched its Atlantis 018 Peripheral Imaging Catheter, which reportedly brings existing 40MHz coronary ...
The FDA has approved the LipiScan Coronary Imaging System, an angiography laser scanner by InfraReDx Inc., which is intended to characterize fatty deposits in coronary vessel walls using near infrared diffuse spectroscopy.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The recently FDA-approved COGNIS CRT-D and the TELIGEN ICD from Boston Scientific are among the smallest and thinnest high-energy devices at 32.5 cc and 31.5 cc respectively, while less than 10 mm thick. Both devices offer extended battery longevity over previous company devices, self-correcting software and improved programming technology.
The landscape of cardiovascular care is changing. In the cath lab alone, the shift in volume and type of diagnostic and interventional procedures has broadened the lab’s scope, increased demand and raised expectations.
September 1, 2008 - Depression and heart disease are the two leading disorders with the strongest contributions to the ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
September 2, 2008 - Merit Medical Systems Inc., a manufacturer of disposable devices used primarily in cardiology and radiology procedures, today said five new products are scheduled to be introduced over the next few months.
September 2, 2008 – The European Society of Cardiology (ESC) released new guidelines for how doctors should handle an acute pulmonary embolism (PE), during its 2008 Congress in Munich, Germany today.
September 3, 2008 - Edwards Lifesciences Corp. today said it received FDA approval for the Carpentier-Edwards PERIMOUNT ...

 September 03, 2008
September 03, 2008




