February 5, 2008 - Doctors completed the first implant of Boston Scientific’s COGNIS cardiac resynchronization therapy defibrillator (CRT-D), which reportedly represents an entirely new platform to treat heart failure.
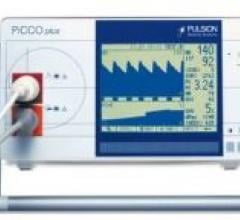
For patient monitoring systems not integrated with PiCCO-Technology, Pulsion Medical Systems offers the PiCCO parameters ...
March 3, 2008 - Boston Scientific Corp. received CE Mark approval of its ACUITY Spiral left ventricular lead for use with cardiac resynchronization therapy defibrillators and cardiac resynchronization therapy pacemakers, marking the company�s seventh U.S. or European regulatory approval in the past nine weeks.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
March 5, 2008 - Edwards Lifesciences Corp. reported that the first three human implants of the next-generation Edwards transcatheter aortic heart valve were performed at St. Paul's Hospital in Vancouver, British Columbia, marking the progression of minimally-invasive valve therapy.
March 4, 2008 - Visage Imaging will unveil its Visage Thin Client solutions portfolio, and demonstrate scalability for ...
The new version of Visage CS Cardiac Analysis is said to feature a set of new tools and optimizations such as calcium scoring, enhanced reporting and manual editing of the left ventricle geometry. Visage CS Cardiac Analysis is designed to allow viewing and post-processing of even the largest multi-phase cardiac CT studies, using Visage’s server-based thin-client performance.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
March 4, 2008 - By using a dual-source technique on a single computed tomography (CT) system, a research team at the ...
March 3, 2008 – Covidien said that it has tentative FDA clearance for its Abbreviated New Drug Application (ANDA) for ...
March 3, 2008 - CardiacQA surpassed 20,000 reports on its Nuclear Report Generator, marking a milestone for the web ...
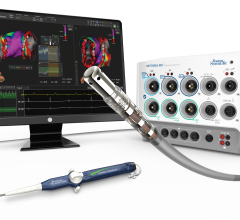
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
March 3, 2008 – The FDA granted 510(k) clearance for the AIGISRX CRMD Anti-Bacterial Envelope by Tyrxpharma, a cardiac rhythm medical device intended to immobilize and reduce bacterial infection of a pacemaker or implantable cardioverter defibrillator (ICD) in order to create a stable environment when implanted in the body.
March 3, 2008 - The FDA, National Heart Lung & Blood Institute and National Science Foundation are hosting a Workshop on Computer Methods for Cardiovascular Devices, in Bethesda, MD, from March 18-19 to address regulatory and technical issues with medical devices.
March 3, 2008 - Vermillion Inc. has entered into an exclusive license agreement with Stanford University to develop and ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Philips' cardiovascular IT solution, the enhanced Xcelera solution is an integrated multimodality solution reportedly provides one access point for all relevant cardiology information, such as cardiovascular ultrasound, CT, MR, nuclear medicine, ECGs and electrophysiology recording signals, as well as long-term storage and enterprise distribution of multimedia studies.
February 29, 2008 – A record-breaking 28,400 people and 900 companies attended the 2008 Annual Healthcare Information ...
McKesson's ECG Management module, a component of Horizon Cardiology, is a fully integrated, Web-enabled ...

 March 04, 2008
March 04, 2008