January 9, 2009 - Cheetah Medical said this week it was granted FDA 510(k) marketing clearance for the noninvasive blood ...
January 9, 2009 - CardioNet Inc. today announced the publication of two studies and the presentation of two abstracts ...
January 8, 2009 - The budgetary constraints facing many healthcare facilities will significantly affect the markets for ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
January 8, 2009 - The American College of Cardiology Foundation (ACCF) and its partners this week released a new ...
January 8, 2009 - The Premier healthcare alliance sent a letter to Congressional leadership yesterday urging the ...
January 7, 2009 - Cameron Health Inc. announced today the first CE trial implants in Europe and New Zealand for Cameron Health’s Subcutaneous Implantable Defibrillator (S-ICD) System.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
January 7, 2009 - The annual "Top 20 Best in KLAS Awards: Software & Professional Services" report named Hyland Software ...
January 7, 2009 - According to a study performed at the Yale University School of Medicine in New Haven, CT, the ...
January 7, 2009 – Every year, more than 500,000 people have coronary bypass surgery, and according to a New England ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
January 7, 2009 - As part of an imaging and storage expansion initiative, Mercy Medical Center, Des Moines, IA, placed ...
January 7, 2009 - The Sixth International Chronic Total Occlusion Summit is Feb. 5-6, at the New York Marriott Marquis ...
Janaury 7, 2009 - The Japanese Ministry of Health, Labour and Welfare (MHLW) granted approval to AGA Medical Corp. for ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
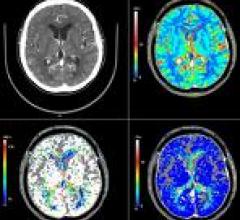
January 6, 2009 - Ziosoft Inc., an advanced visualization and analysis software for medical imaging, today received 510(k) clearance from the FDA for its CT brain perfusion application, which is accessible to clinicians throughout the enterprise using the Ziostation thin-client system.
January 6, 2009 - New research published in the January edition of the HeartRhythm Journal reveals that abandoning a ...
January 6, 2009 - Vicor Technologies received FDA 510k approval in December to market its PD2i Analyzer, which uses a ...

 January 08, 2009
January 08, 2009