October 13, 2014 — After reviewing updated data and analysis for the Boston Scientific Watchman left atrial appendage (LAA) closure device, the U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel of the Medical Devices Advisory Committee voted in favor of the device. By a vote of 6 to 5 (with 1 abstention) the panel concluded the benefits of the Watchman device outweigh the potential risks.
Developed for practices and clinics that do not have a PACS or other disaster recovery solution, Informity uses cloud-based technology to automatically and continuously back up patient information, images and system settings on the ImagePilot Sigma and ImagePilot Aero. It assures business continuance with a one button restore of the entire system.
A report released by the Department of Health and Human Services projects that hospitals will save $5.7 billion this year in uncompensated care costs because of the Affordable Care Act, with states that have expanded Medicaid seeing about 74 percent of the total savings nationally compared to states that have not expanded Medicaid.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
A study using a new imaging tool, optical coherence tomography (OCT), offered a new look at the composition of carotid artery disease and has the potential to alter how physicians understand and treat the disease, a leading cause of stroke.
Although cardiovascular disease is largely avoidable through lifestyle modifications, it remains the nation’s number one cause of death. While annual wellness exams offer physicians the chance to advise patients on modifying cardiac risk factors, that advice can easily get lost given the amount of information covered during a routine check-up.
“Once patients have survived cancer, they don't die from cancer, they die from heart disease. Cardio-oncology is about making sure that doesn't happen," said Juan Carlos Plana, M.D.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
C. R. Bard Inc. announced the U.S. Food and Drug Administration (FDA) approval of the Lutonix 035 drug-coated balloon (DCB) catheter for percutaneous transluminal angioplasty (PTA) after pre-dilatation, for the treatment of de novo or restenotic lesions up to 150 mm in length in native vascular disease of the superficial femoral or popliteal arteries with reference vessel diameters of 4 mm to 6mm.
October 10, 2014—Mitralign Inc. reported on the successful use of its technology to perform a percutaneous repair on a patient with tricuspid regurgitation (TR). Prof. Dr. med. J. Schofer of the Medicare Center and Department for Percutaneous Interventions of Structural Heart Disease, Albertinen Heart Center, Hamburg, Germany; and Rebecca Hahn, M.D., director of interventional echocardiography, Columbia University Medical Center / New York Medical Center / New York Presbyterian Hospital, presented at the PCR London Valves Conference and detailed the procedure—a percutaneous bicuspidization of the tricuspid valve, successfully converting a regurgitating tri-leaflet valve into a functioning bi-leaflet valve. The German regulatory body BfArM approved the patient for a compassionate use exemption, as no other options were available. The successful procedure was performed at the Albertinen Heart Center in Hamburg. The Mitralign product is currently being evaluated in clinical trials for an indication in functional mitral regurgitation. The device is not approved for sale or distribution.

October 10, 2014 — Despite ongoing commercial challenges in 2013, the global medtech industry’s financial performance held steady at the relatively low levels of growth that have become common in recent years. But even as the industry grapples with these market and regulatory pressures, it faces a potential growing challenge: the threat of commoditization, according to new findings outlined in EY’s annual medical technology report, Pulse of the industry: differentiating differently, released today.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
A KLAS report revealed a lack of widespread integration with EMRs continues to limit the effectiveness of order sets and care plans.
Direct Flow Medical Inc., a transcatheter heart valve innovator focused on improving patient outcomes has received the CE Mark for an enhanced transfemoral delivery system for the Direct Flow Medical Transcatheter Aortic Valve System.
The swift migration to cloud computing and data recovery services shows no signs of slowing, with half of organizations reporting they expect to boost their cloud services budgets over the next 18 months, according to a new study conducted by IDG Research Services on behalf of Sungard Availability Services and EMC Corp.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
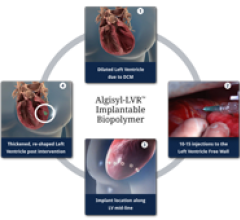
LoneStar Heart Inc. announced that it received the CE mark (Conformite Europeene) for its Algisyl-LVR hydrogel implant, the company's lead product for the treatment of advanced heart failure (HF).
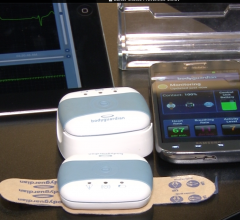
Preventice Inc. announced improvements to its BodyGuardian Remote Monitoring System that have the potential to simplify the remote monitoring experience for patients and contribute to a more efficient care delivery process when transitioning between monitoring needs for individual patients.
CardiacAssist Inc. announced that the first Protek Duo veno-venous extracorporeal life support (VV ECLS) kit was utilized Sept. 24, at Memorial Hermann Hospital in Houston.


 October 13, 2014
October 13, 2014