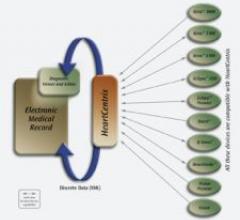
Cardiac Science Corp. has launched HeartCentrix, a software solution that enables its diagnostic stress, Holter and electrocardiography devices to communicate seamlessly with physician office-based electronic medical record (EMR) systems.
St. Jude Medical Inc. says it has received FDA approval of a new cardiac rhythm management device designed to help physicians manage heart failure (HF) patients, including patients who have or may develop atrial fibrillation (AF).
A new MRI monitor to complement the Maglife product line, the Maglife light makes MRI monitoring within reach of virtually every MRI Center. It can handle all monitoring needs and is compatible with MRI scanners up to 3T.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
When used in conjunction with the ev3 embolic protection device, ev3’s PROTÉGÉ RX Carotid Stent has been FDA cleared for the treatment of carotid artery disease in patients who are at high-risk for adverse events from carotid artery surgery. The cleatance was supported by the CREATE (Carotid Revascularization with ev3 Inc.
VIASYS Healthcare is showcasing the launch of the MasterScreen CPX, a product that combines 40 years of CPET experience into a compact unit.
Pfizer says the FDA has approved the company’s Lipitor (atorvastatin calcium) Tablets to reduce the risk of nonfatal heart attacks, fatal and non-fatal strokes, certain types of heart surgery, hospitalization for heart failure, and chest pain in patients with heart disease — making Lipitor the first cholesterol-lowering medication to receive FDA approval for the reduction of the risk of hospit
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
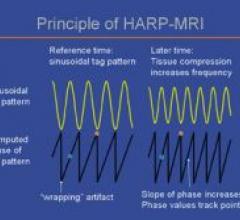
Diagnosoft HARP is software that assists physicians in the analysis of magnetic resonance (MR) images by providing quantitative measurements and visualization of regional heart function. Based on technology from Johns Hopkins University, it’s the first FDA-cleared software designed for the analysis of tagged MR images.
The NICO2 Respiratory Profile Monitor is designed to monitor the patient side of the breathing circuit. NICO2 goes beyond conventional capnography to measure breath-by-breath volumetric CO2 and takes the guesswork out of ventilation management from setup to weaning.
March 22 2007 — ZOLL Medical Corp announced today it received FDA clearance to market and sell the ZOLL M Series with ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The modern world of medicine has been revolutionized by the inception of minimally invasive interventional techniques ...
March 22, 2007 — St. Jude Medical Inc. says it has received FDA approval of a new cardiac rhythm management device ...
March 22, 2007 — Evalve, Inc., a privately held medical device company, has announced the FDA has approved a registry of up to 70 patients who are at high risk of mortality from surgical treatment for mitral regurgitation (MR) as part of its ongoing pivotal EVEREST Study (Endovascular Valve Edge-to-Edge REpair STudy).
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Cardiac exams represent only about five percent of all CT procedures, but cardiac CT continues to generate the most interest at manufacturers’ exhibits as seen at the 2006 RSNA Annual Scientific Meeting. The fascination with Cardiovascular Computed Tomography (CVCT) remains high even though full reimbursement is not expected to occur until 2009, according to industry observers at RSNA.
March 22, 2007 — Pfizer says the FDA has approved the company’s Lipitor (atorvastatin calcium) Tablets to reduce the ...
March 22, 2007 — Edwards Lifesciences Corp. says it has received conditional FDA approval to initiate a pivotal clinical ...

 March 22, 2007
March 22, 2007