The recently FDA-approved COGNIS CRT-D and the TELIGEN ICD from Boston Scientific are among the smallest and thinnest high-energy devices at 32.5 cc and 31.5 cc respectively, while less than 10 mm thick. Both devices offer extended battery longevity over previous company devices, self-correcting software and improved programming technology.

The use of embolic protection devices (EPD) during percutaneous cardiac procedures has helped reduce the number of complications due to debris being released into the bloodstream and causing blockages in smaller vessels. The devices are designed to capture and remove debris that may be dislodged during procedures. There are three classes of EPD devices:
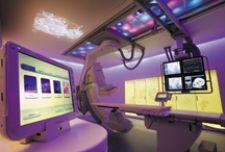
In as much as the environment in which you live can affect your quality of life, so can the environment in which someone receives medical care affect patient outcome – at least in theory.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Spectranetics Corp. recently announced the availability of its LLD EZ Lead Locking Device (LLD) for the removal of ...
TRUMPF Medical Systems Inc. offers an in-light high-definition (HD) operating room camera, the TruVidia HD, which ...
St. Jude Medical’s Promote RF CRT-D (cardiac resynchronization therapy defibrillator) and Current RF ICD (implantable cardioverter defibrillator) are radiofrequency (RF) wireless devices used to treat patients with heart failure and with potentially lethal heart arrhythmias.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
September 3, 2008 - CardioNet Inc. said today Humana upgraded the CardioNet System to a “covered benefit” status, from ...

If the case went to court for whether positron emission tomography (PET) or single, photon emission computed tomography (SPECT) is better suited for myocardial perfusion imaging, experts say the current technology and developments on the horizon would result in a hung jury.
Cook Medical recently launched the EVOLUTION-Shortie Mechanical Dilator Sheath Set, a tool for venous entry during ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Carestream Health’s CARESTREAM Virtual 3D Solution is a 3D software package designed to deliver advanced 3D processing ...
Medtronic’s Concerto/Virtuoso line of implantable cardiac devices feature Medtronic’s proprietary Conexus Wireless Telemetry, developed using the Medical Implant Communications Service (MICS). Using the MICS band enables reliable communication between the implanted device and clinician programmers and patient home monitoring units.
September 3, 2008 – Research published this week in the New England Journal of Medicine said patients with heart failure in whom an ICD is implanted and receive shocks for any arrhythmia have a substantially higher risk of death than similar patients who do not receive such shocks.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
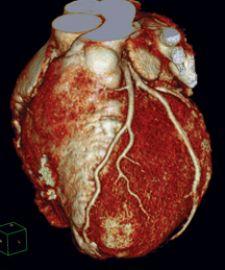
The landscape of cardiovascular care is changing. In the cath lab alone, the shift in volume and type of diagnostic and interventional procedures has broadened the lab’s scope, increased demand and raised expectations.
LUMEDX Corp. offers the CardioPACS 5.0, the latest version of its cardiology PACS software. The multimodality, Web ...
Cook Medical has received approval from the FDA for its Zenith TX2 Thoracic TAA Endovascular Graft, marking the expansion of Cook Medical’s portfolio of devices to treat aortic diseases.

 September 03, 2008
September 03, 2008




