Biosensors International has announced enrollment of the first patient in LEADERS FREE, a clinical study involving BioFreedom, a polymer-free drug-coated stent (DCS) from Biosensors.
Fovia Medical Inc. and Blackford Analysis Ltd. announced plans to deliver compatible SDKs to the medical advanced visualization market.
Accumetrics Inc. announced that its next generation VerifyNow II System has achieved CE marking for point-of-care measurement of platelet reactivity to antiplatelet agents. This marks the culmination of a series of important milestones in 2012, increasing evidence of the clinical value of platelet reactivity testing and significantly expanding the market opportunities for the VerifyNow System in both surgical and interventional cardiology patient populations.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Biotronik announced that the European Heart Journal has published the clinical results of the ECOST trial (Effectiveness and Cost of ICD Follow-Up Schedule with Telecardiology). The randomized controlled study evaluated the safety and efficacy of Biotronik Home Monitoring compared with standard in-office follow-up visits for patients with implantable cardioverter-defibrillators (ICDs).
January 3, 2013 — Cardiovascular Systems Inc. (CSI) announced it has completed enrollment in its ORBIT II clinical trial, enrolling 443 patients. ORBIT II is evaluating the safety and effectiveness of the company’s orbital atherectomy technology in treating severely calcified coronary arteries.
January 3, 2013 — BioVentrix announced it has received CE mark for its Revivent myocardial anchoring system, which makes possible Less Invasive Ventricular Enhancement (LIVE), a procedure that restores the left ventricle from damage done by a heart attack to a more optimal volume and conical shape.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Miracor Medical Systems GmbH announced that its PICSO system was used for the first time under CE mark in the United Kingdom to treat a patient with a large acute heart attack, or ST-segment elevation myocardial infarction (STEMI). Interventional cardiologist Dr. Magdi El-Omar performed the PICSO procedure in Manchester, England.
Tensys Medical Inc has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the TL-300, the company’s latest generation system. The TL-300 is a member of Tensys’ established T-Line family, which has successfully been used by over 50,000 patients since commercial launch. The T-Line technology accurately and continuously captures a patient’s beat-to-beat waveform and blood pressure in a completely noninvasive fashion, providing physicians with a stream of real-time hemodynamic data that is not possible using traditional noninvasive blood pressure (cuff) devices. Avoiding the blind-time associated with a deflated or inaccurate cuff can enhance hemodynamic monitoring, which has been definitively linked to improved clinical outcomes.
The Society of Cardiovascular Computed Tomography (SCCT) announced the publication of the SCCT Expert Consensus Document on Computed Tomography Imaging Before Transcatheter Aortic Valve Implantation (TAVI)/Transcatheter Aortic Valve Replacement (TAVR) in the Journal of Cardiovascular Computed Tomography (JCCT).
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
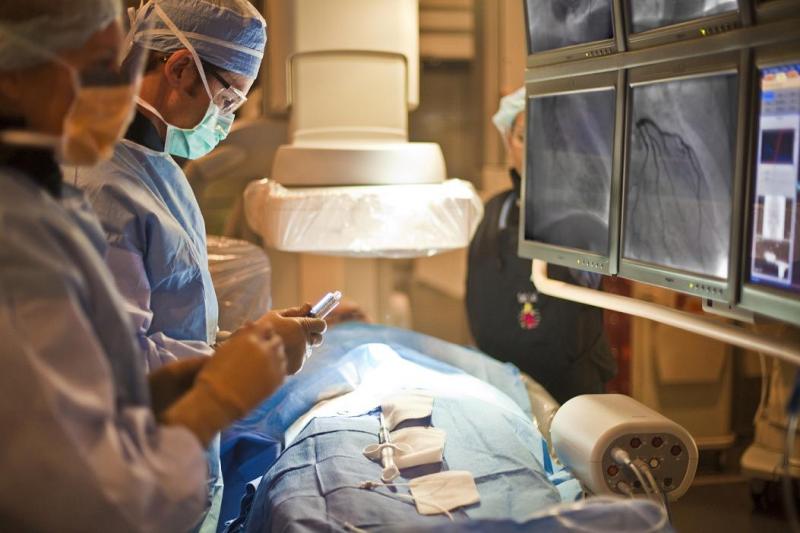
A report from the CathPCI Registry, a data registry that includes information from 85 percent of the heart catheterization laboratories in the United States, is providing a vivid snapshot of the current practice of invasive cardiology.

Abbott announced that the Xience Xpedition everolimus-eluting coronary stent system received U.S. Food and Drug Administration (FDA) approval and is launching immediately in the United States, providing physicians with a next-generation technology with the largest size matrix in the U.S. market.
The Medical Imaging and Technology Alliance (MITA) said that failure to delay the new medical device excise tax, along with Medicare cuts for imaging and radiation therapy services passed by Congress as part of the “fiscal cliff” package, will hinder patients’ access to early disease detection and therapy services and threaten American medical technology jobs.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
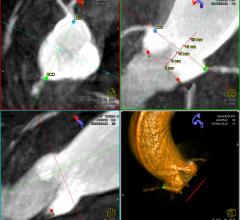
Siemens Healthcare recently received clearance from the U.S. Food and Drug Administration (FDA) for syngo Aortic ValveGuide, an integrated image processing software that helps cardiologists and cardiac surgeons prepare and perform transcatheter aortic valve replacement (TAVR). The syngo Aortic ValveGuide automatically reconstructs a 3-D representation of the aortic root from cross-sectional images acquired with the angiography system and provides the best projection angle for the valve replacement. The software selects anatomical landmarks and overlays the 3-D image with 2-D images acquired during live fluoroscopy, enabling the physician to obtain real-time 3-D guidance in the patient’s body while navigating the new valve to its intended location. This groundbreaking software, which is now available to customers, reflects the spirit of Siemens Healthcare’s Agenda 2013 — a recently announced two-year initiative designed in part to strengthen the Healthcare Sector's innovative power and competitiveness.
Philips Royal Electronics and Unfors RaySafe AB announced the signing of a Joint Development Agreement that will see the development of the next generation Philips DoseAware system, with the aim of offering seamless access and communication between Philips DoseAware and Philips Allura interventional X-ray systems.
At RSNA 2012 Montage Healthcare Solutions and Radimetrics demonstrated the benefits of correlating dose information with Montage clinical report search results.

 January 07, 2013
January 07, 2013