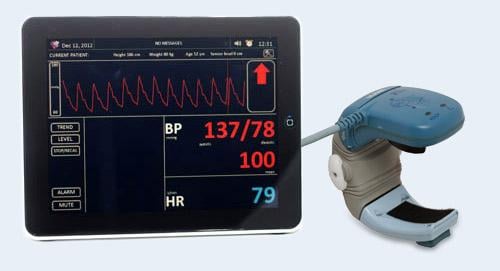
January 3, 2013 — Tensys Medical Inc has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the TL-300, the company’s latest generation hemodynamic monitoring system. The TL-300 is a member of Tensys’ established T-Line family, which has successfully been used by over 50,000 patients since commercial launch. The T-Line technology accurately and continuously captures a patient’s beat-to-beat waveform and blood pressure in a completely noninvasive fashion, providing physicians with a stream of real-time hemodynamic data that is not possible using traditional noninvasive blood pressure (cuff) devices. Avoiding the blind-time associated with a deflated or inaccurate cuff can enhance hemodynamic monitoring, which has been definitively linked to improved clinical outcomes.
The TL-300 features a tablet-supported monitor that allows touch screen control and displays a patient’s key hemodynamic parameters. In addition to continuous beat-to-beat blood pressure and arterial waveform, the operator is readily able to view trend data for up to a 12-hour period. The tablet displays data coming from an integrated bracelet, sensor and wrist frame placed over the patient’s radial artery. The wrist frame is disposed at the end of the case and the bracelet and sensor are immediately available for the next patient. Today, the T-line family is used in a number of clinical environments including the operating room, intensive care unit (ICU) and electrophysiology lab.
“The TL-300 represents our continued commitment to delivering next generation, non-invasive, hemodynamic monitoring tools,” commented Oliver Goedje, Tensys’ medical director. “This new system will not only enhance our current product offering, but will serve as a platform as we add cardiac output and additional hemodynamic parameters in the near future.”
Tensys will be providing additional details regarding the TL-300 launch and other company initiatives at the upcoming 2013 OneMedForum in San Francisco, Calif. Tensys management will be presenting on Jan. 9 at 10:10AM (presentation room Franciscan).
For more information: www.tensysmedical.com


 November 14, 2025
November 14, 2025 









