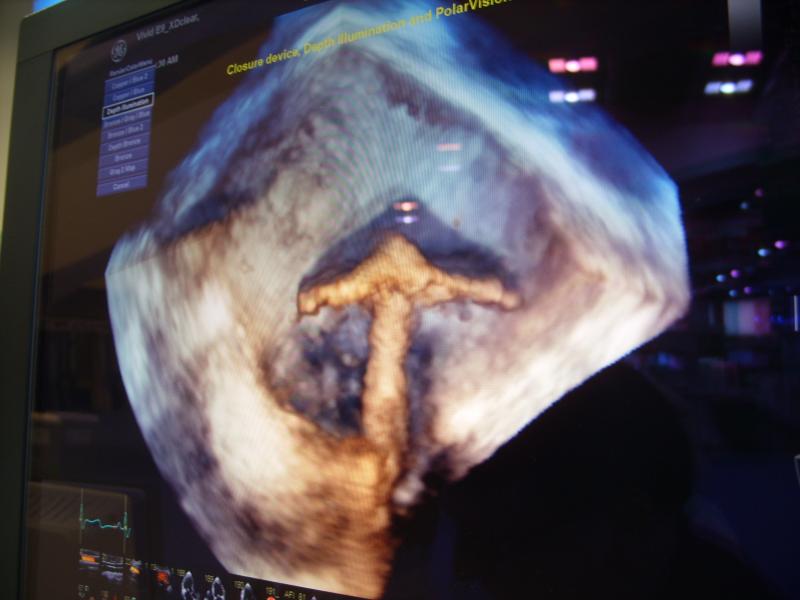
Transesophageal echocardiography (TEE) is quickly establishing itself as an essential tool in the interventional cardiologist’s daily toolbox. An alternative to the more traditional transthoracic echo, TEE provides higher quality images due to the esophagus’ location directly behind the heart. With the introduction of 3-D/4-D TEE, the technology has become a primary imaging modality to guide new, complex transcatheter structural heart procedures, including transcatheter aortic valve repair (TAVR), MitraClip, transcatheter mitral valve replacements (TMVR), left atrial appendage (LAA) occlusions, and septal defect closures.
Vital Images Inc. announced the release of VioSuite Image Management and Vitality Solutions Business Intelligence, giving healthcare administrators a robust set of tools to improve system-wide image access and image data analytics. Vital will introduce the VioSuite and Vitality Solutions portfolios at the 2015 Healthcare Information and Management Systems Society annual conference (HIMSS15), April 13-15 in Chicago.
An expert consensus statement released by four leading cardiovascular societies provides new guidance on percutaneous mechanical circulatory support (MCS) devices for treatment of heart failure. The statement — jointly released by the Society for Cardiovascular Angiography and Interventions (SCAI), American College of Cardiology (ACC), Heart Failure Society of America (HFSA) and The Society of Thoracic Surgeons (STS) —aims to help physicians match the right device with the right patient.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
IMRIS Inc. announced that Visius iCT, its ceiling-mounted intraoperative computed tomography scanner, has received Health Canada licensing allowing for sales and marketing in the country.
Bradford Teaching Hospitals NHS Foundation Trust (Bradford) has completed one of the UK’s largest image migration projects thanks to a four-way partnership alongside healthcare data management specialists BridgeHead Software, Dell and Agfa.
Boston-based medical imaging developer Paxeramed Corp. will introduce its PaxeraUltima360° enterprise-class and vendor neutral platform during the 2015 Healthcare Information and Management Systems Society (HIMSS) annual conference. The platform can consolidate medical image data from multiple imaging departments into a master directory reaching radiologists, clinicians and patients.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Royal Philips announced that Volcano, a Philips business, has received CE Mark for the iFR Scout pullback software. iFR Scout is a functional extension of its existing instant wave-Free Ratio (iFR) Modality, optimized to assess serial lesions and diffuse coronary disease.
Two new studies suggest women who experience hot flashes earlier in life appear to have poorer endothelial function — one of the earliest signs of cardiovascular disease— than those who have hot flashes later in life or not at all. Both studies were presented at the American College of Cardiology’s 64th Annual Scientific Session in San Diego.
The opening session speakers at the Society for Cardiovascular Angiography and Interventions (SCAI) 2015 Scientific Sessions will examine the current state of cardiovascular care and why it is ripe for disruption and new technologies.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Agfa HealthCare announced that it will be launching its new clinical Portal in North America. The Agfa HealthCare Portal gives a patient-centric overview of information from different sources, to different stakeholders in the patient's care, inside or outside the hospital. It will also provide a comprehensive road map running from the Portal itself to true integrated care and the electronic health record (EHR).
TeraMedica will introduce a sophisticated analytics platform and dashboard for its vendor neutral archive (VNA) at the 2015 Healthcare Information and Management Systems Society (HIMSS) annual conference.
PatientPoint announced that PatientPoint Outreach 3.2 is now certified for Meaningful Use in an ambulatory setting. The software is also compliant with the Office of the National Coordinator (ONC) 2014 Edition criteria for certified electronic health record technology (CEHRT).
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Patients 65 or older discharged from the hospital as early as 48 hours after angioplasty following a major heart attack have similar outcomes as those who stay four to five days, provided there are no in-hospital complications. This finding was published in the Journal of the American College of Cardiology.
The Virginia Zoo veterinary team partnered with a team from Sentara Heart Hospital to perform ultrasound on the hearts of orangutans Pepper and Schnitz as part of the animals’ annual wellness checkups at the Zoo’s state-of-the-art veterinary hospital.
Agfa HealthCare will unveil enhancements to its suite of technologies during the Healthcare Information and Management Systems Society (HIMSS) annual conference that are designed to integrate multiple points of data consolidation and access with its Enterprise Imaging platform. The new capabilities will be on display at the HIMSS15 Annual Conference & Exhibition, April 12-16, 2015, in Chicago.

 April 08, 2015
April 08, 2015