The recently FDA-approved COGNIS CRT-D and the TELIGEN ICD from Boston Scientific are among the smallest and thinnest high-energy devices at 32.5 cc and 31.5 cc respectively, while less than 10 mm thick. Both devices offer extended battery longevity over previous company devices, self-correcting software and improved programming technology.
As the new editor of Diagnostic and Invasive Cardiology (DAIC), I hope communication will not be one-sided with me presenting or reviewing new technology on the market. I would like to hear from the readers — the end users of these products — to find out your thoughts. I would also like to find out what you consider as important issues, or what technologies you would like to hear more about.
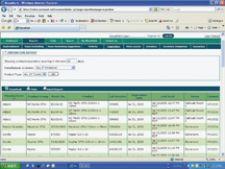
Tight inventory control in the cath and electrophysiology (EP) labs is critical to hospitals' bottom lines due to the expense of the items and their rapid expirations. A new technology showing big returns on investment is radio-frequency identification (RFID) tracking.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
September 3, 2008 – Research published this week in the New England Journal of Medicine said patients with heart failure in whom an ICD is implanted and receive shocks for any arrhythmia have a substantially higher risk of death than similar patients who do not receive such shocks.
Spectranetics Corp. recently announced the availability of its LLD EZ Lead Locking Device (LLD) for the removal of ...
TRUMPF Medical Systems Inc. offers an in-light high-definition (HD) operating room camera, the TruVidia HD, which ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
St. Jude Medical’s Promote RF CRT-D (cardiac resynchronization therapy defibrillator) and Current RF ICD (implantable cardioverter defibrillator) are radiofrequency (RF) wireless devices used to treat patients with heart failure and with potentially lethal heart arrhythmias.
This comparison chart features three categories of stents: coronary, carotid and peripheral. We gratefully acknowledge ...
Cook Medical recently launched the EVOLUTION-Shortie Mechanical Dilator Sheath Set, a tool for venous entry during ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Carestream Health’s CARESTREAM Virtual 3D Solution is a 3D software package designed to deliver advanced 3D processing ...
Medtronic’s Concerto/Virtuoso line of implantable cardiac devices feature Medtronic’s proprietary Conexus Wireless Telemetry, developed using the Medical Implant Communications Service (MICS). Using the MICS band enables reliable communication between the implanted device and clinician programmers and patient home monitoring units.
The process of the coronary CT angiography (CTA) becoming the preferred imaging modality is akin to the process of obtaining a pilot’s license.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
September 3, 2008 – While a recent study into whether using unselected and selected bone-marrow derived cells can help with regeneration of heart tissue did not lead to significant improvement of LVEF and LV volumes in comparison to control group, there was a trend toward significant improvement of LVEF in patients with severely depressed baseline LVEF receiving either MNC or CD34 CXCR4 bone ma
LUMEDX Corp. offers the CardioPACS 5.0, the latest version of its cardiology PACS software. The multimodality, Web ...
Cook Medical has received approval from the FDA for its Zenith TX2 Thoracic TAA Endovascular Graft, marking the expansion of Cook Medical’s portfolio of devices to treat aortic diseases.

 September 03, 2008
September 03, 2008




