Cook Medical has received approval from the FDA for its Zenith TX2 Thoracic TAA Endovascular Graft, marking the expansion of Cook Medical’s portfolio of devices to treat aortic diseases.
St. Jude Medical Inc. recently released its EnSite System Version 8.0, software designed to intuitively visualize the ...

The use of embolic protection devices (EPD) during percutaneous cardiac procedures has helped reduce the number of complications due to debris being released into the bloodstream and causing blockages in smaller vessels. The devices are designed to capture and remove debris that may be dislodged during procedures. There are three classes of EPD devices:
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Nihon Kohden America Inc. said its Prefense Early Detection and Notification System received 510(k) clearance from the ...
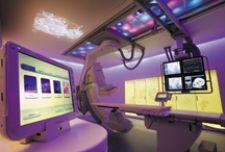
In as much as the environment in which you live can affect your quality of life, so can the environment in which someone receives medical care affect patient outcome – at least in theory.
September 3, 2008 - CardioNet Inc. said today Humana upgraded the CardioNet System to a “covered benefit” status, from ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
The FDA recently cleared Boston Scientific Corp.’s PROMUS Everolimus-Eluting Coronary Stent System and Abbott Vascular’s ...
AngioScore Inc. has introduced longer and larger AngioSculpt PTA Scoring Balloon Catheters for the treatment of peripheral artery disease (PAD).
The R Series defibrillator for hospitals aims for simplicity and operational readiness to help improve in-hospital ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

If the case went to court for whether positron emission tomography (PET) or single, photon emission computed tomography (SPECT) is better suited for myocardial perfusion imaging, experts say the current technology and developments on the horizon would result in a hung jury.
September 3, 2008 - Edwards Lifesciences Corp. today said it received FDA approval for the Carpentier-Edwards PERIMOUNT ...
September 2, 2008 - The safety debate about drug-eluting stents (DES) is still ongoing and in these times of uncertainty ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
September 1, 2008 - Depression and heart disease are the two leading disorders with the strongest contributions to the ...
Edwards Lifesciences Corp. produces the Carpentier-Edwards The PERIMOUNT Magna mitral valve was launched in Europe in September 2005. It incorporates features of the Carpentier-Edwards PERIMOUNT mitral valve, which has demonstrated 16 years of durability, including the treatment of the bovine pericardial tissue leaflets with the Carpentier-Edwards ThermaFix process. September 2008
September 2, 2008 - Merit Medical Systems Inc., a manufacturer of disposable devices used primarily in cardiology and radiology procedures, today said five new products are scheduled to be introduced over the next few months.

 September 03, 2008
September 03, 2008




