
The use of embolic protection devices (EPD) during percutaneous cardiac procedures has helped reduce the number of complications due to debris being released into the bloodstream and causing blockages in smaller vessels. The devices are designed to capture and remove debris that may be dislodged during procedures. There are three classes of EPD devices:
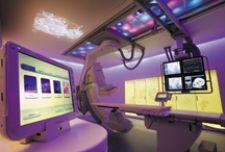
In as much as the environment in which you live can affect your quality of life, so can the environment in which someone receives medical care affect patient outcome – at least in theory.
September 3, 2008 - CardioNet Inc. said today Humana upgraded the CardioNet System to a “covered benefit” status, from ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
LUMEDX Corp. offers the CardioPACS 5.0, the latest version of its cardiology PACS software. The multimodality, Web ...
Cook Medical has received approval from the FDA for its Zenith TX2 Thoracic TAA Endovascular Graft, marking the expansion of Cook Medical’s portfolio of devices to treat aortic diseases.
St. Jude Medical Inc. recently released its EnSite System Version 8.0, software designed to intuitively visualize the ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Nihon Kohden America Inc. said its Prefense Early Detection and Notification System received 510(k) clearance from the ...

If the case went to court for whether positron emission tomography (PET) or single, photon emission computed tomography (SPECT) is better suited for myocardial perfusion imaging, experts say the current technology and developments on the horizon would result in a hung jury.
September 3, 2008 – Research published this week in the New England Journal of Medicine said patients with heart failure in whom an ICD is implanted and receive shocks for any arrhythmia have a substantially higher risk of death than similar patients who do not receive such shocks.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
September 2, 2008 - The safety debate about drug-eluting stents (DES) is still ongoing and in these times of uncertainty ...
September 1, 2008 - Depression and heart disease are the two leading disorders with the strongest contributions to the ...
September 2, 2008 - Merit Medical Systems Inc., a manufacturer of disposable devices used primarily in cardiology and radiology procedures, today said five new products are scheduled to be introduced over the next few months.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Edwards Lifesciences Corp. produces the Carpentier-Edwards The PERIMOUNT Magna mitral valve was launched in Europe in September 2005. It incorporates features of the Carpentier-Edwards PERIMOUNT mitral valve, which has demonstrated 16 years of durability, including the treatment of the bovine pericardial tissue leaflets with the Carpentier-Edwards ThermaFix process. September 2008
September 2, 2008 – The European Society of Cardiology (ESC) released new guidelines for how doctors should handle an acute pulmonary embolism (PE), during its 2008 Congress in Munich, Germany today.
September 3, 2008 - Edwards Lifesciences Corp. today said it received FDA approval for the Carpentier-Edwards PERIMOUNT ...

 September 03, 2008
September 03, 2008




