July 10, 2008 - Boston Scientific Corp. this week launched its Atlantis .018 Peripheral Imaging Catheter, which reportedly brings existing 40 MHz coronary standard ultrasound imaging resolution to peripheral interventions. The company said the product is available for immediately release in the U.S.
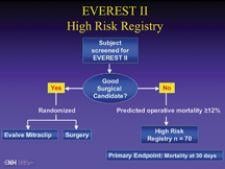
Preliminary results of a multicenter study show that the use of tiny clips to repair the mitral valve may someday ...
July 10, 2008 - Datascope Corp. said today it exercised its option to acquire the Peripheral Vascular Stent business of ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The only safe assumption to make about today’s current gold standard imaging modality used to identify and evaluate atheromatous plaque in the peripheral arteries is that there is not one gold standard. To locate and evaluate these plaques that cause peripheral vascular disease (PVD), physicians have multiple tried-and-true imaging tests at their disposal.
July 10, 2008 - A novel blood pool magnetic resonance angiography (MRA) agent, Vasovist (gadofosveset trisodium), has ...
InTouch Technologies Inc. offers StrokeRESPOND to extend the functionality of its telemedicine, Remote Presence robotic ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Thoratec Corp. received FDA approval of its PMA (premarket approval) application, allowing the use of its HeartMate II LVAS (left ventricular assist system) as a bridge-to-transplantation (BTT) in patients suffering from advanced-stage heart failure.
Northeast Monitoring’s DR200/HE is a combination 14-day Holter plus 30-day Event recorder integrated into a single unit ...
July 9, 2008 – The Medical Imaging & Technology Alliance (MITA) today applauded the U.S. Congress for passing Medicare ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The FDA cleared GE Healthcare’s new LightSpeed CT750 HD, said to be the world’s first high-definition CT scanner that ...
Vascular Insights received 510(k) clearance from the FDA to market its ClariVein infusion catheter for infusion of physician-specified agents in the peripheral vasculature. ClariVein is a percutaneous, 2 2/3 Fr (0.035-inch) catheter, containing a rotating wire driven by a motor, that enhances fluid dispersion in the treatment area.
July 9, 2008 - InnerSpace has added the V-Series line of heavy-duty cabinets, carts and workstations to its organized ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The Abiomed Inc. Impella 2.5 cardiac assist device was FDA cleared in 2008 for use for partial circulatory support for periods up to six hours. The intra-aortic balloon pump (IABP) also has 510(k) clearance and approximately 110,000 are used each year in the U.S.
The FloTrac is reportedly the first minimally invasive hemodynamic monitoring device that connects to any existing arterial line and requires no manual calibration. The latest enhancement to the FloTrac software empowers clinicians with greater flexibility to trend and analyze the patient parameters in order to make better informed decisions, said the company.
July 9, 2008 - New research shows catheter cryoablation is comparably effective to radiofrequency catheter ablation for the treatment of atrial flutter (AFL) and may have some safety advantages over the use of radiofrequency.

 July 09, 2008
July 09, 2008