July 23, 2008 — Vascular Solutions, Inc. recently launched the Axis Wire, a 0.035-inch specialty guidewire that combines ...
July 23, 2008 – A recent study found intensive LDL-cholesterol lowering with the combination of the drugs simvastatin ...
July 22, 2008 - CyGene Laboratories Inc. this week is introducing StrokeScan, a genetic screening test aimed at identifying high risk individuals who have a family history of stroke, cardiovascular or kidney disease.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
July 22, 2008 - Barco won a notable order for its Coronis Fusion 6MP DL diagnostic display system under a PACS installation project in the Beatrix Hospital in Gorinchem, the Netherlands, as part of a larger PACS installation program carried out by Philips Healthcare.
July 22, 2008 – To recognize excellence in the use of healthcare information technology, McKesson yesterday announced the winners of its eighth annual VIP Award competition, which included Provincial Health Contact Centre, Winnipeg, Manitoba for its program to better manage patients with chronic diseases, specifically congestive heart failure.
July 22, 2008 - Osmetech today said it received FDA 510(k) clearance for its eSensor Warfarin Sensitivity Test to be used as an aid in the identification of patients at risk for increased sensitivity to the widely used blood-thinning drug, warfarin. The company also said the FDA clearance includes its second-generation eSensor XT-8 molecular diagnostics platform.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
July 21, 2008 – Toshiba America Medical Systems said today that four live cases using the Infinix CF-i/BP will be showcased at the Pediatric and Adult Interventional Cardiac Symposium (PICS-AICS) held in Las Vegas July 20-23.
July 18, 2008 - A Federal lawsuit filed in Birmingham, Alabama July 18 charges pharmaceutical company Actavis Totowa ...
July 17, 2008 – A new study reveals that, with dual-source computed tomography (DSCT), the dosage for a heart ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
July 21, 2008 – The Premier healthcare alliance today became the first group purchasing organization (GPO) to endorse ...
July 17, 2008 - A new study reveals that, with dual-source computed tomography (DSCT), the effective dosage for a heart examination can be significantly lowered, in comparison to conventional CT. The study also demonstrated that stenoses can be diagnosed with similar high accuracy as with invasive X-ray angiography.
July 17, 2008 - Omnicell Inc. and St. Patrick Hospital and Health Sciences Center in Missoula, MT, today said they installed an interface between the hospital’s Omnicell OptiFlex CL bar code inventory tracking system and its Philips Series IV physiomonitoring and information system.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
July 17, 2008 - Heart sounds provide valuable diagnostic information concerning the heart valves and hemodynamics of heart, but the poor sensitivity of human ears in the low frequency range makes this task difficult, so two researchers have created a computer-aided phonocardiogram (PCG) to analyze a digitized recordings of heart sounds.
July 16, 2008 - Earlier this week President Bush vetoed the Medicare Improvements for Patients and Providers Act (HR 6331) because it excluded a 10.6 percent pay cut to doctors who treat patients in the Medicare program. However, on July 15 the U.S. House of Representatives and Senate voted overwhelmingly voted to override the veto.
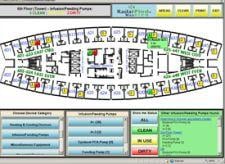
Clinicians spend a lot of time walking around hospitals looking for equipment, other staff members, and sometimes even their patients.

 July 22, 2008
July 22, 2008




