The FDA cleared GE Healthcare’s new LightSpeed CT750 HD, said to be the world’s first high-definition CT scanner that ...
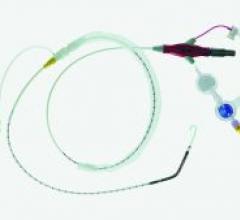
Vascular Insights received 510(k) clearance from the FDA to market its ClariVein infusion catheter for infusion of physician-specified agents in the peripheral vasculature. ClariVein is a percutaneous, 2 2/3 Fr (0.035-inch) catheter, containing a rotating wire driven by a motor, that enhances fluid dispersion in the treatment area.
July 10, 2008 - The XIV Congress of the Latin American Society of Interventional Cardiology (SOLACI) and the annual ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The Abiomed Inc. Impella 2.5 cardiac assist device was FDA cleared in 2008 for use for partial circulatory support for periods up to six hours. The intra-aortic balloon pump (IABP) also has 510(k) clearance and approximately 110,000 are used each year in the U.S.
The FloTrac is reportedly the first minimally invasive hemodynamic monitoring device that connects to any existing arterial line and requires no manual calibration. The latest enhancement to the FloTrac software empowers clinicians with greater flexibility to trend and analyze the patient parameters in order to make better informed decisions, said the company.
July 9, 2008 – The American Heart Association recently issued new guidelines for computed tomography (CT) and magnetic ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
July 8, 2008 - Foothills Medical Centre, a leading medical centre for treating patients with cardiac rhythm abnormalities in Calgary, Alberta, has become the first facility in Canada to use the new Evolution Mechanical Dilator Sheath Set from Cook Medical for the removal of pacemaker leads, reportedly simplifying this potentially challenging medical procedure.
TRUMPF Medical Systems Inc. offers an in-light high definition (HD) operating room camera, the TruVidia HD, which captures and transmits razor sharp, true-to-life images. The camera offers a 1,080 line horizontal resolution and 2 million pixels. The images are said to be more than twice as clear as those available from standard definition cameras.
July 8, 2008 - Expanding the application of endovascular aortic repair, Medtronic Inc. today announced two major ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
July 8, 2008 - The University of Mississippi Medical Center and St. Peter’s Hospital each implemented a suite of IT ...
July 7, 2008 - The American Academy of Pediatrics (AAP) today issued new cholesterol screening and treatment ...
July 7, 2008 - OrbusNeich today said data recently presented at the 17th Annual Meeting of the Japanese Society of ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
July 7, 2008 - InTouch Technologies Inc. today launched a new product feature, StrokeRESPOND, to extend the ...
July 3, 2008 - A unanimous federal jury in Texas recently found that two patents exclusively licensed to Pressure Products Medical Supplies Inc., which cover medical products called valved peel-away introducers used for placement and implantation of pacemaker leads, were valid and infringed by the FlowGuard and ViaSeal products marketed by Quan Emerteq Corp.
July 3, 2008 - A multicenter trial with up to five years of follow-up continues to support the mid- and long-term durability and safety of the Zenith endovascular graft used for aneurysm repair.


 July 08, 2008
July 08, 2008