InTouch Technologies Inc. offers StrokeRESPOND to extend the functionality of its telemedicine, Remote Presence robotic ...
Thoratec Corp. received FDA approval of its PMA (premarket approval) application, allowing the use of its HeartMate II LVAS (left ventricular assist system) as a bridge-to-transplantation (BTT) in patients suffering from advanced-stage heart failure.
Northeast Monitoring’s DR200/HE is a combination 14-day Holter plus 30-day Event recorder integrated into a single unit ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
July 9, 2008 – The Medical Imaging & Technology Alliance (MITA) today applauded the U.S. Congress for passing Medicare ...
The FDA cleared GE Healthcare’s new LightSpeed CT750 HD, said to be the world’s first high-definition CT scanner that ...
Vascular Insights received 510(k) clearance from the FDA to market its ClariVein infusion catheter for infusion of physician-specified agents in the peripheral vasculature. ClariVein is a percutaneous, 2 2/3 Fr (0.035-inch) catheter, containing a rotating wire driven by a motor, that enhances fluid dispersion in the treatment area.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
July 8, 2008 - The University of Mississippi Medical Center and St. Peter’s Hospital each implemented a suite of IT ...
TRUMPF Medical Systems Inc. offers an in-light high definition (HD) operating room camera, the TruVidia HD, which captures and transmits razor sharp, true-to-life images. The camera offers a 1,080 line horizontal resolution and 2 million pixels. The images are said to be more than twice as clear as those available from standard definition cameras.
July 8, 2008 - Foothills Medical Centre, a leading medical centre for treating patients with cardiac rhythm abnormalities in Calgary, Alberta, has become the first facility in Canada to use the new Evolution Mechanical Dilator Sheath Set from Cook Medical for the removal of pacemaker leads, reportedly simplifying this potentially challenging medical procedure.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
July 8, 2008 - Expanding the application of endovascular aortic repair, Medtronic Inc. today announced two major ...
July 7, 2008 - OrbusNeich today said data recently presented at the 17th Annual Meeting of the Japanese Society of ...
July 7, 2008 - InTouch Technologies Inc. today launched a new product feature, StrokeRESPOND, to extend the ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
July 7, 2008 - The American Academy of Pediatrics (AAP) today issued new cholesterol screening and treatment ...
July 3, 2008 - A multicenter trial with up to five years of follow-up continues to support the mid- and long-term durability and safety of the Zenith endovascular graft used for aneurysm repair.
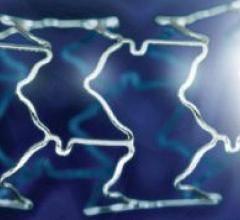
Boston Scientific Corp. offers the PROMUS Everolimus-Eluting Coronary Stent System for the treatment of coronary artery disease. The PROMUS Stent is a private-labeled XIENCE V Everolimus-Eluting Coronary Stent System manufactured by Abbott and distributed by Boston Scientific under an agreement executed prior to the 2006 acquisition of the former Guidant Corp. by Boston Scientific.


 July 08, 2008
July 08, 2008