According to a statement in Health Data Management, Ochsner Health System in New Orleans, developed a program utilizing a Carematix Blipcare wireless weight scale for remote patient monitoring to cut readmissions of heart failure patients by 40 percent.
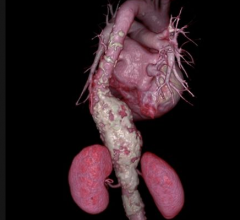
A 19-year-old woman was successfully implanted with the SynCardia temporary Total Artificial Heart and bridged to a donor heart transplant after cardiac surgeons used 3-D virtual implantation to determine she was fit-eligible for the procedure.
A coordinated emergency response by healthcare teams to treat heart attack patients meant faster care that was associated with improved survival, according to research presented at the American Heart Association’s Scientific Sessions 2014.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Agfa HealthCare will showcase its updated Enterprise Imaging Xero Viewer at RSNA 2014.
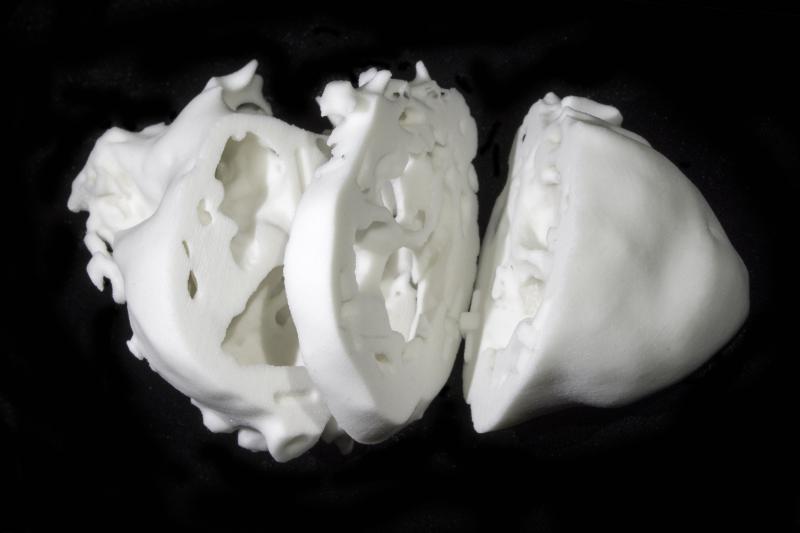
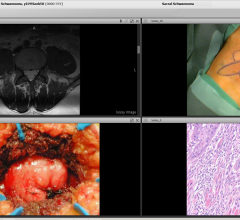
An experimental 3-D printed model of the heart may help surgeons treat patients born with complicated heart disorders, according to research presented at the American Heart Association’s Scientific Sessions 2014.
Oxford University researchers presented data that reveal the extent to which smoking causes silent but deadly damage to health at the annual scientific meeting of the American Heart Association (AHA).
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
The new ResolutionMD functionality, which will be showcased at the 2014 RSNA conference by Calgary Scientific, offers better access to health information, supports better teamwork and enhances communication among practitioners and patients.
The Centers for Medicare & Medicaid Services (CMS) released final rules outlining how Medicare will pay major health care providers and suppliers in 2015.
At the 2014 Radiological Society of North America (RSNA) meeting, Cerner Corp. will feature RadNet Radiation Dose Documentation functionality.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Carestream will debut its Carestream Touch Ultrasound System that offers a combination of enhanced user experience and image quality to meet the needs of even the most demanding radiologists and sonographers. The product release also marks Carestream’s entry into the ultrasound market.
Routinely adding mitral valve repair to coronary artery bypass graft surgery for heart attack patients may not be warranted in patients with moderate mitral valve damage, according to an NIH-funded study.
Cleveland Clinic announced its 9th annual list of Top 10 Medical Innovations that are likely to have major impact on improving patient care in 2015. The list includes a mobile stroke ambulance, fast, painless blood testing and a novel intra-operative radiation approach for breast cancer.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
November 19, 2014 — Gagnon Cardiovascular Institute at Morristown Medical Center has become the first facility in New ...
Vital Images Inc. announced enhancements in image management to its VitreaView enterprise viewer with VioStream software. Today, many healthcare organizations are challenged to unify access to imaging content, often spread across a number of departmental picture archive and communication systems (PACS) and distributed locations. Vital is now offering building blocks which will enable full image access through electronic health records (EHRs).
CIRS will feature a variety of quality assurance (QA), research and training phantoms for all radiology imaging modalities at the 2014 Radiological Society of North America (RSNA) meeting, held Nov. 30-Dec. 3 in Chicago.


 November 24, 2014
November 24, 2014