Ekos Corp., a BTG International group company, published results of its Ultrasound Accelerated Thrombolysis of Pulmonary Embolism (PE) trial (ULTIMA) in the peer-reviewed journal of the American Heart Association, Circulation. The announcement was made at the International Symposium on Endovascular Therapy (ISET), Miami, Fla.
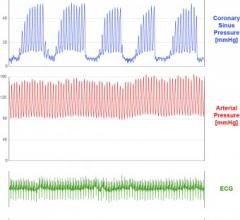
Miracor Medical Systems completed four months’ follow-up of patients enrolled in the “Prepare RAMSES” clinical trial of PICSO (Pressure-controlled Intermittent Coronary Sinus Occlusion) System.
Heart transplant patients may live 20 years or more after surgery, according to a study in The Annals of Thoracic Surgery. Hector Rodriguez Cetina Biefer, M.D. and Markus J. Wilhelm, M.D., from the University Hospital Zurich in Switzerland, led a research team that examined long-term outcomes in 133 patients from their institution who underwent heart transplantation from 1985 to 1991.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
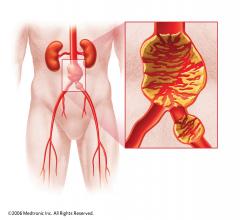
W. L. Gore & Associates Inc. (Gore) and AAAneurysm Outreach have partnered to raise awareness and drive screening of individuals at-risk of abdominal aortic aneurysm (AAA).
Ascendian Healthcare Consulting announced a service line to assist clients with planning, design, development and implementation of Cardiovascular Information Systems (CVIS).

Expanded use of percutaneous coronary intervention (PCI) is seeing more patients with acute coronary syndromes treated more quickly, according to the latest National Audit of PCI (covering 2012).
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

New leaders, new categories, new Best in KLAS segments and an aethetic overhaul are some of the highlights of the 2013 Best in KLAS Awards: Software & Services report.
The Centers for Medicare and Medicaid Services (CMS) released findings on a number of its initiatives to reform the healthcare delivery system. These include interim financial results for select Medicare accountable care organization (ACO) initiatives, an in-depth savings analysis for Pioneer ACOs, results from the physician group practice demonstration, and expanded participation in the bundled payments for care improvement initiative. Savings from both the Medicare ACOs and Pioneer ACOs exceed $380 million.
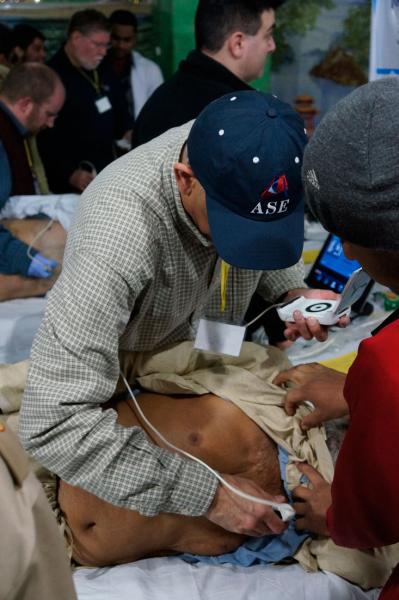
A paper in the journal of the World Heart Federation, Global Heart, reported mounting evidence of the utility of ultrasound in areas outside its traditional field of cardiology, with increasing use reported in general hospital wards, clinics and pre-hospital environments. The paper is by Associate Professor Bret Nelson and Dr. Amy Sanghvi, Mount Sinai School of Medicine, New York.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Biotronik enrolled the first patients in an expansion of their ongoing ProMRI trial to test its pacemaker system.Phase A of the study evaluated the safety of current Biotronik pacemaker systems during magnetic resonance imaging (MRI) scans with exclusion zones of the chest area, and was completed on November 18, 2013.
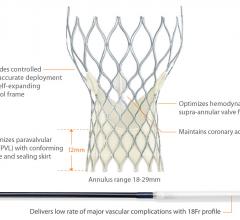
The Gagnon Cardiovascular Institute at Morristown Medical Center adopted a minimally invasive medical device to treat patients with severe aortic stenosis who are too ill or frail to have their aortic valves replaced trough traditional open-heart surgery.
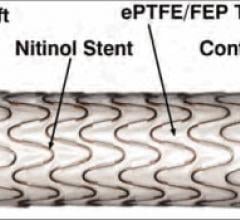
January 28, 2014 — The U.S. Food and Drug Administration (FDA) approved W. L. Gore & Associates’ 25 cm Gore Viabahn Endoprosthesis with Heparin Bioactive Surface for the treatment of symptomatic peripheral arterial disease (PAD) lesions in the Superficial Femoral Artery (SFA). The 25 cm endoprosthesis is designed to cover long-segment lesions in the SFA, potentially reducing the need for multiple devices.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
ReCor Medical advanced its Paradise System for renal denervation (RDN) for treatment resistant hypertension (HTN).
The U.S. Food and Drug Administration (FDA) approved Pressure Products Inc.’s SafeSept Needle Free Transseptal Guidewire for use with any introducer system when crossing the interatrial septum. The device assists in transseptal procedures and is designed to create the primary puncture in the interatrial septum providing access from the right to left side of the heart without using a transseptal needle.
AtheroMed, a developer of catheter technologies for treating peripheral artery disease (PAD), received clearance from the U.S. Food and Drug Administration (FDA) to market the Phoenix Atherectomy System, which allows physicians to treat PAD with a low profile atherectomy catheter that continuously removes diseased material as it is debulked and does not require specialized capital equipment.

 February 03, 2014
February 03, 2014