January 28, 2014 — The U.S. Food and Drug Administration (FDA) approved W. L. Gore & Associates’ 25 cm Gore Viabahn Endoprosthesis with Heparin Bioactive Surface for the treatment of symptomatic
peripheral arterial disease (PAD) lesions in the Superficial Femoral Artery (SFA). The 25 cm endoprosthesis is designed to cover long-segment lesions in the SFA, potentially reducing the need for multiple devices.
The FDA approval of the 25 cm device was supported by evidence from a multicenter European
trial. Patients enrolled in the study had lesion lengths ranging from 20 to 40 cm, with a mean length of 26.5 cm. Of all patients enrolled, 92.9 percent had total occlusions of the SFA. One-year data demonstrated a freedom from target lesion revascularization of 78.2 percent. In addition, the Gore Viabahn device exhibited no fractures at 12 months.
The 25 cm study results supplement data from a physician-sponsored investigational device exemption (IDE) trial conducted previously.
“We have been awaiting the approval of the 25 cm [Gore Viabahn device] since our study directly comparing the [Gore Viabahn] Stent-graft as an endoluminal bypass in the SFA to open femoral popliteal bypass of the SFA was completed,” said Dennis Gable, M.D., vascular surgeon at The Heart Hospital Baylor Plano, Plano, Texas. “Our results demonstrated no difference in primary patency or limb salvage out to four years when comparing the two treatment modalities—even with a mean stented length of 25.6 cm in the [Gore Viabahn device] arm. Our study results are supported by the similar outcomes of the European data and show that treatment of truly long lesions can now successfully be accomplished with only one stent device with excellent results.”
The
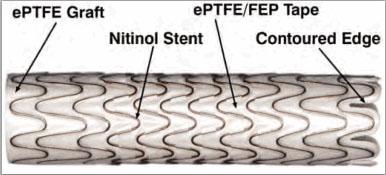
stent-graft design of the Gore Viabahn Endoprosthesis with Heparin Bioactive Surface consists of a durable, reinforced, biocompatible, ePTFE liner attached to an external nitinol stent structure that acts as a barrier to in-stent restenosis. The device is available in configurations that are compatible with 0.035 in or 0.018/0.014 in wire platforms. The device also incorporates the Heparin Bioactive Surface, which utilizes a proprietary end-point covalent immobilization of heparin to the surface of the endoprosthesis. This proprietary surface technology is intended to provide a thromboresistant surface through sustained heparin bioactivity.
For more information: www.goremedical.com