Boston Scientific Corporation has begun the United States and European launch of its Victory guidewire, designed to facilitate crossing of resistant lesions and the placement and exchange of balloon catheters or other interventional devices within the peripheral vasculature. The company expects to launch the product in other international markets later this year and in 2013, subject to regulatory approvals.
New research from the Netherlands suggests tiny microbubbles can be used to more effectively deliver a blood clot busting drug to patients while they are in the ambulance during acute heart attack.
The U.S. Food and Drug Administration (FDA) has granted Boston Scientific Corp. regulatory approval for its S-ICD System, the world's first commercially available subcutaneous implantable cardioverter defibrillator (S-ICD). It sits entirely just below the skin without the need for implantable lead to be placed inside the heart.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
October 8, 2012 — Simbionix USA Corp. will introduce the new advanced U/S (ultrasound) Mentor simulator at the upcoming American College of Emergency Physicians (ACEP) Exhibition in Denver, Colo. This new Simbionix simulator offers realistic hands-on training for diverse ultrasound examinations and interventions.
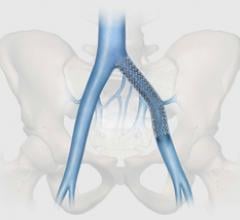
Following Health Canada approval, Cook Medical has made the Zilver Vena Venous Self-Expanding Stent available to physicians across Canada. Designed to restore blood flow in obstructed iliofemoral veins, Zilver Vena provides physicians with a tool designed specifically for stenting obstructed iliofemoral veins.
October 8, 2012 — St. Jude Medical Inc. has launched MediGuide Technology, the first 3-D navigation system intended for the evaluation of vascular and cardiac anatomy on a recorded fluoroscopic image instead of live fluoroscopy. The use of recorded images allows physicians to reduce the duration of radiation exposure during cardiovascular procedures, especially during long procedures in the electrophysiology (EP) lab.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The U.S. Food and Drug Administration (FDA) has granted Clinical Laboratory Improvement Amendments (CLIA)-waived status to the Roche CoaguChek XS Plus system. The point-of-care anticoagulation monitor offers connectivity and data management tools to help healthcare professionals manage PT/INR (blood clotting time) testing. The waiver means that the monitoring technology may now be used in a broader range of clinical settings, such as labs that do not meet the requirements to perform moderate- or high-complexity testing as defined by the CLIA of 1988.
Imagine a place where doctors can tell patients in advance if cancer treatment will work for them, without going through an entire course of chemotherapy.
Advocate Health Care, one of the nation's top health systems and the largest integrated health care system in the state of Illinois, and GE Healthcare, a national leader in low dose, high performance imaging, announced a joint effort to help further reduce radiation dose in computed tomography (CT). The goal is to optimize care for patients needing imaging procedures and reduce radiation where possible without adversely impacting image quality. It’s one of the first announcements of the GE Blueprint for low dose, a comprehensive campaign in which GE Healthcare is working alongside leading U.S. health systems to further reduce radiation dose in CT imaging. Leaders from Advocate and GE Healthcare unveiled the Advocate-based GE Blueprint for low dose today during an event at Advocate Lutheran General Hospital.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
LipoScience Inc., a highly innovative diagnostic company that is advancing patient care by developing high value proprietary blood tests using nuclear magnetic resonance (NMR) technology, announced the publication of results from a clinical study in the American Journal of Cardiology (AJC) suggesting that despite having cholesterol levels considered acceptable by today’s clinical standards, patients with Type 2 diabetes mellitus (T2DM) are at increased risk for cardiovascular disease (CVD)-related events.
The American Medical Association (AMA) Current Procedural Terminology (CPT 1) Editorial Panel has assigned Category I CPT codes specifically for bronchial thermoplasty in its recently published CPT 2013 Professional Edition. Beginning Jan. 1, 2013, physicians and hospitals will be able to seek reimbursement through two new codes to describe the bronchial thermoplasty procedure. Category I CPT procedure codes are recognized by all public and private health insurance payers in the United States.
Researchers at the University of Maryland School of Medicine, who are exploring novel ways to treat serious heart problems in children, have conducted the first direct comparison of the regenerative abilities of neonatal and adult-derived human cardiac stem cells. Among their findings: cardiac stem cells (CSCs) from newborns have a three-fold ability to restore heart function to nearly normal levels compared with adult CSCs. Further, in animal models of heart attack, hearts treated with neonatal stem cells pumped stronger than those given adult cells. The study is published in the Sept. 11, 2012, issue of Circulation.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Aptus Endosystems Inc. announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its thoracic-length HeliFX Aortic Securement System. Similar to the original HeliFX system that was cleared in November 2011 and designed for treating abdominal aortic aneurysms (AAA), the new system consists of a longer delivery device with additional tip configurations to bring the innovative helical EndoAnchor technology to the treatment of thoracic aortic aneurysms (TAA).
This year’s European Society of Cardiology (ESC) Congress in Munich, Germany, was a stark example of exactly where the cardiovascular market space is headed. This congress fully emphasized the new and innovative technologies that are coming out on the medical device and medical technology side, while leaving the market’s pharmaceutical wing in the dust. In fact, out of the nine hours over three days and 18 seminars on cardiac therapy innovation, only one seminar, given by Amgen, spoke about a pharmaceutical interventional treatment for heart disease. Thus, if there is one message to take away from the ESC 2012 congress, it is that the pharmaceutical industry has moved away from the cardiovascular market
The National Institutes of Health (NIH)/National Cancer Institute (NCI) has awarded a five-year research project grant (R01) to identify harmonized reconstruction parameters for each currently-produced positron emission tomography (PET)/computed tomography (CT) scanner for use in clinical trials. The Society of Nuclear Medicine and Molecular Imaging’s (SNMMI) Clinical Trials Network (CTN) will serve as an administrative coordinating body for the Image Reconstruction Harmonization Grant, which was awarded in August to the Universities of Iowa, Pennsylvania, and Washington.


 October 11, 2012
October 11, 2012