October 29, 2010 – A range of enhancements to an advanced visualization suite will be shown at RSNA 2010. Shina Systems will display its virtual 3Di suite, which delivers imaging data, reformatting and viewing tools and powerful computer processing in a cloud environment.
The CX50 integrated ultrasound system, by Philips, provides premium ultrasound image quality for enhanced guidance and ...
XperGuide, by Philips, offers live 3-D image needle guidance by overlaying live fluoroscopy and 3-D soft tissue imaging ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
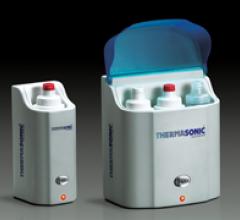
Two Thermasonic gel warmers, by Parker Laboratories, will be introduced at RSNA 2010. The first, a three-bottle gel ...
October 28, 2010 – A new medical appropriateness decision support solution will be on display at RSNA 2010. OrderRight 2.0, by MedCurrent, gives primary care and specialty physicians the information they need at the time of the radiologic test so the appropriate tests can be ordered.
December 1, 2010 – A completely Web-based picture archiving and communication system (PACS) is on display at the RSNA 2010 meeting. Infinitt PACS is hardware-agnostic and offers an embedded 3-D PACS viewer and a full array of image visualization and management tools. Several hospitals in the United States are switching to the company’s PACS system because of the savings it offers.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
October 28, 2010 - The first patent related to specific human blood protein variant biomarkers related to cardiovascular disease has been issued. The United States Patents and Trademark Office has issued U.S. Patent 7,816,095 for Intrinsic Bioprobes covering novel blood protein biomarkers related to cardiovascular disease.
October 28, 2010 – A report by the American College of Cardiology (ACC) provides new criteria for selecting patients who could benefit from cardiac computed tomography (CCT). The report, which was developed in partnership with the Society of Cardiovascular Computed Tomography (SCCT) also informs payers about appropriate clinical scenarios for its use.
October 28, 2010 - Coronary stent manufacturer Stentys is now listed and traded on the NYSE Euronext Paris (Ticker: STNT). As a result of the IPO, the company has raised $31.9 million, which will be used to commercialize its stents in Europe and obtain regulatory approvals in the United States.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
October 22, 2010 – To help clinicians better understand meaningful use in imaging, TeraMedica has created the Vendor Neutral Archive (VNA) University online educational program.
Bracco offers contrast imaging agents for computed tomography (CT), magnetic resonance imaging (MRI), angiography and ...
MediCal QAWeb, by Barco, is the first online service for high-grade quality assurance (QA). The all-inclusive secured ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The ProScribe, by Barco, is an advanced mobile point-of-care display for physicians and nurses who need to stay ...
The CliniScape mobile clinical assistant, by Barco, enables nurses and physicians to make more informed clinical ...
October 27, 2010 – A new digital silicon photomultiplier technology has been developed with applications in both X-ray and nuclear imaging. Philips developed the technology by scaling a single-pixel sensor to a fully integrated 64-pixel sensor with a sensing surface of more than 10 square centimeters.

 October 29, 2010
October 29, 2010