January 13, 2009 - Stereotaxis Inc. said the first crossing of a chronic total occlusion (CTO) using the RF PowerAssert ...
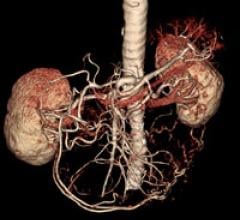
January 13, 2009 - Vital Images Inc. and Toshiba Medical Systems Corp. have agreed to collaborate on a joint investment ...
January 13, 2009 - Micell Technologies, a development-stage biomedical device company dedicated to developing innovative interventional cardiology systems, has obtained the rights to Maxcor's CE-marked Genius MAGIC Cobalt Chromium Coronary Stent System for the purpose of developing and marketing drug-eluting stents (DES) based on Micell's prop coating technology.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
January 13, 2009 - Edwards Lifesciences Corp. said Jan. 9 the Patents Court in the United Kingdom upheld the validity of ...
January 12, 2009 - Influenza, strep throat and the common cold afflict many people during the winter months, yet viral ...
January 12, 2009 - Medtronic Inc. today said it entered into an agreement whereby Medtronic will acquire Ablation ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
January 8, 2009 - Two RSNA exhibits featuring Aquarius iNtuition by TeraRecon won Cum Laude awards at the recent 94th annual Radiological Society of North America (RSNA) Scientific Assembly at McCormick Place in Chicago, IL, Nov. 30 - Dec. 5, 2008.
January 9, 2009 - The Heart Hospital of Austin is the first hospital in the world to use the new Stereotaxis RF ...
January 9, 2009 - Cheetah Medical said this week it was granted FDA 510(k) marketing clearance for the noninvasive blood ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
January 9, 2009 - CardioNet Inc. today announced the publication of two studies and the presentation of two abstracts ...
January 9, 2009 - TeraRecon Inc. revealed yesterday that two exhibits featuring Aquarius iNtuition won Cum Laude awards ...
January 9, 2009 - Cardiac nuclear medicine company Positron Corp. said today it will market its complete turnkey ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
January 8, 2009 - The budgetary constraints facing many healthcare facilities will significantly affect the markets for ...
January 8, 2009 - The American College of Cardiology Foundation (ACCF) and its partners this week released a new ...
January 8, 2009 - The Premier healthcare alliance sent a letter to Congressional leadership yesterday urging the ...

 January 12, 2009
January 12, 2009