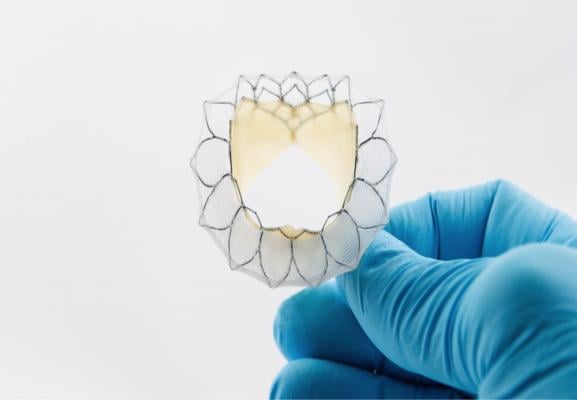
The Neovasc Tiara transcatheter mitral valve is currently being tested in U.S. and international clinical trials.
(This article was updated in June 2018)
The advent of transcatheter valve repair and replacement technologies is one of those rare instances where there is a rapid paradigm shift in medicine. Since the first transcatheter aortic valve replacement (TAVR) systems were cleared by the U.S. Food and Drug Administration (FDA) in 2011 (Edwards Lifesciences Sapien) and 2014 (Medtronic CoreValve), TAVR rapidly expanded. It is also fueling enthusiasm for new transcatheter mitral valve replacement (TMVR) technology.
In 2014, the number of U.S. TAVR procedures according to the STS/ACC Transcatheter Valve (TVT) Registry was 15,834 implants. The number for 2015 is estimated to be on track for about 18,000 procedures, according to John Carroll, M.D., co-medical director, Cardiac and Vascular Center, University of Colorado Hospital, who has been tracking TVT statistics. As of September, he said there were 396 sites performing TAVR in the United States.
The Medtronic CoreValve pivotal trial data has shown CoreValve is superior to surgical valve replacement in high-risk patients. The most recent data from the Sapien 3 device showed the TAVR device was on par with surgery and resulted in the lowest recorded mortality and stroke rates ever reported in a TAVR study, said Martin Leon, M.D., director, Center for Interventional Vascular Therapy, Columbia University Medical Center/New York-Presbyterian Hospital, at the Transcatheter Cardiovascular Therapeutics (TCT) annual meeting in October.
“What has changed has been very dramatic in the past few years,” Leon said. “This has been a breakthrough therapy, and a decade ago I don’t think any of us would have believed that there could have been a breakthrough with valves.”
He said the improved outcomes are due to a combination of the simplification of the devices used, better designs to improve TAVR valve performance, and improved procedural performance as operators gain more experience. He said the minimally invasive nature of TAVR also holds future promise to become an out-patient procedure. Leon said Hopital du Sacre-Coeur de Montreal, performed its first same-day TAVR procedure in May 2015.
TAVR is only indicated for very sick, high-risk patients, but with new FDA-pending indications for intermediate risk patients on the horizon, Leon said TAVR is set to expand much further than its current use. “About 80 percent of the patients we treat for aortic regurgitation today are intermediate risk,” he said.
Read the article “First TAVR Device Receives European Approval to Treat Intermediate Risk Patients” from August 2016.
With the rapid improvement in TAVR outcomes to match or outperform surgery begs the question of why TAVR is not considered for a wider patient population and if surgery will remain the gold standard in the coming years. “We have been asking the question of who qualifies for TAVR, but we will turn that premise on its head and ask who will benefit most from TAVR, not who will benefit most from surgery,” Leon said. “In the future, the vast majority of patients will be treated with TAVR — maybe 80 to 90 percent.”
Watch the video “The Evolution of TAVR Technology.” Interview with Juan Granada, M.D., executive director and chief scientific officer of the Cardiovascular Research Foundation's Skirball Center for Innovation, at the Transcatheter Valve Therapies 2015 meeting.
New TAVR Systems Introduced
In June, the FDA cleared two new, improved versions of the only two TAVR systems on the U.S. market.
Medtronic released its recapturable, self-expanding CoreValve Evolut R System, which is the first recapturable and repositionable device available in the United States. Its EnVeo R Delivery System features an InLine Sheath that significantly reduces the profile to a 14 French equivalent to enable wider use of the preferred transfemoral access route. The new valve comes in 23, 26 and 29 mm sizes.
The Edwards Lifesciences’ Sapien 3 valve with the Commander Delivery System has a fabric skirt at its base to better seal it against the annulus to help eliminate paravalvular leak. Clinical data show improvements over the pervious generation Sapien devices, with a disabling stroke rate of only 2 percent and only 2.3 percent of patients had moderate paravalvular leaks, and no major leaks reported. The valve is comes in 20, 23, 26 and 29 mm sizes.
Watch the video “TAVR Beats Surgery — Top News From ACC.16.” Dr. Vinod Thourani, professor of surgery, Division of Cardiothoracic Surgery, Department of Surgery, Emory University School of Medicine and a co-investigator for the PARTNER II Trial, discusses the biggest news item from ACC.16 — the Sapien 3 TAVR device performed better that surgical aortic valve replacement.
Watch the video “CoreValve Trumps Surgical Valve Replacement — TVT 2015.” Interview with Michael Reardon, M.D., professor of cardiothoracic surgery at DeBakey Heart and Vascular Center, and chairman of the patient screening committee, CoreValve U.S. pivotal trial, at the Transcatheter Valve Therapies 2015 meeting.
FDA Clears Transcatheter Valve-In-Valve Procedures
Both the CoreValve and the Sapien valves were recently granted additional FDA indications for valve-in-valve (VIV) implants for high-risk and extreme risk patients. The CoreValve was the first to receive the new indication in March. The Sapient XT valve received the indication in October. The VIV indication allows the implantation of a TAVR device into a failing surgically implanted tissue aortic valve replacement valve. When a surgical valve degenerates over time, patients may require another valve replacement. However, some patients are too sick or frail for a second open-heart surgery and the transcatheter VIV procedure now provides a new treatment option.
The Sapien XT TAVR device VIV data presented at TCT showed a high overall one-year survival rate of 86.6 percent and a low overall stroke rate of 3.7 percent in a very high-risk patient population, according to the 197-patient PARTNER II VIV study.
“This is becoming the preferred method to perform VIV replacements as opposed to surgical replacements, because it is safer than open heart surgery,” said Ajay Kirtane, M.D., chief academic officer, Center for Interventional Vascular Therapy, Columbia University Medical Center/New York-Presbyterian.
Read the article "FDA Clears Sapien XT for Valve-In-Valve Procedures."
Read the article "FDA Expands Use of CoreValve for Aortic Valve-in-Valve Replacement."
Leaflet Issues Uncovered in TAVR
In September 2014, St. Jude Medical stopped its FDA pivotal trial for its Portico TAVR system after valve leaflet thickening was discovered. It was soon discovered that this phenomena is common with all TAVR valves and the trial was restarted in June. The Edwards Lifesciences Fortis transcatheter mitral valve feasibility trial in Europe also was temporarily suspended after a similar finding of thrombus formation on the valve leaflets.
Sessions at EuroPCR in June showed computed tomography (CT) and echo imaging found abnormalities with the valves, including leaflet thickening, impaired leaflet motion and thin films believed to be thrombus, in 7-15 percent of patients. It was also found that these abnormalities were not unique to a particular surgical or transcatheter valve. Most of the abnormalities resolved with the use of oral anticoagulants, but there are no established drug, dose or patient selection protocols. “It is an interesting finding, but we don’t know what the significance is,” Leon said.
Experts in TCT sessions questioned use of anticoagulation therapy, especially in elderly patients, due to increased bleeding complications. TAVR anticoagulation was discussed at many TCT sessions, and there appears to be wide variation in protocols at centers, from warfarin to dual-antiplatelet therapy.
Read more about the valve leaflet issue from the 2017 ACC presentation of the RESOLVE Trial.
Transcatheter Mitral Valves are the Next Frontier
Most interventional and cardiac surgical experts say TMVR will be the next frontier in minimally invasive structural heart interventions. With the success and rapid growth of TAVR, there is an immense anticipation that TMVR will have an even greater impact in cardiology. This has translated into more than $2.5 billion being spent in the past year by vendors purchasing start-up TMVR companies, while less than 50 patients have actually been treated using these technologies, said Michael Mack, M.D., medical director, cardiovascular surgery, Baylor Health Care System and chairman of The Heart Hospital Baylor Plano Research Center.
However, the mitral valve involves much more complex anatomy than the aortic valve, so the devices, imaging for procedural planning and guidance will be much more sophisticated than what is used for TAVR. Among the challenges are: fixation of a device to the very small landing zone of the mitral annulus; avoiding the left ventricular outflow tract (LVOT); avoiding compression of the atrioventricular (AV) node; avoiding the papillary muscle and chordae tendineae; ensuring the device seals properly to avoid paravalvular regurgitation; and the device needs to be able to adapt to remodeling of the anatomy. There are more than 20 TMVR devices in development. The majority of these valves utilize a self-expanding nitinol frame that engages both sides of the native mitral valve annulus for fixation, similar to Amplatzer septal closure devices.
The companies with first-in-human TMVR implants include Tendyne, Neovasc and Edwards Lifesciences’ Fortis and Sapien XT devices. The Neovasc Tiara, Tendyne Bioprosthetic Mitral Valve and CardiAQ Valve Technologies TMVR system all have been granted FDA conditional investigational device exemption (IDE) studies.
Watch the video “Transcatheter Mitral Valve Therapies in Development.”
Large Vendors Buy Up Mitral Start-ups
In mid-October, Boston Scientific closed on an additional round of financing with TMVR start-up MValve Technologies Ltd. Boston Scientific has provided the company with funding since 2012 and has an option to acquire MValve. The cash will be used to fund a first-in-human clinical trial for the MValve docking system, in which the Boston Scientific TAVR Lotus Valve is deployed inside.
Medtronic announced in August it entered an agreement to acquire TMVR start-up Twelve Inc. for $408 million, and an additional $50 million on achievement of European CE mark.
In August, Abbott Vascular invested in two TMVR start-ups. Abbott purchased Tendyne Holdings Inc. for $250 million, plus potential future payments tied to regulatory milestones. Tendyne’s mitral valve has an FDA IDE feasibility study that is currently enrolling patients. There also are plans to begin a clinical trial to support CE mark in Europe.
In a separate transaction, Abbott has provided capital and secured an option to purchase Cephea Valve Technologies. The TMVR start-up was created by several leading cardiologists. Financial terms were not disclosed.
In July, Edwards Lifesciences acquired CardiAQ Valve Technologies Inc. The company has received an FDA IDE approval to conduct an early feasibility study of up to 20 patients, and also plans to initiate a CE mark study in Europe, for its TMVR system. The purchase price is up to $400 million, including $350 million in cash at closing, and the remainder payable upon achievement of a European regulatory milestone.
Edwards already has its own Fortis TMVR device in an early feasibility trial in Europe, but the company said the purchase will offer complementary technology. The Fortis trial was temporarily suspended in May due to thrombus formation on the valve leaflets. The company is working with investigators to change protocols so the trial can restart.
Transcatheter Repair of the Mitral Valve
“There are a myriad on pathological conditions that can change the treatment approach,” Mack said. He explained a patient may have degenerative mitral regurgitation (MR) or functional MR caused by the remodeling the heart. Some patients may fare better with repair versus replacement. The MitraClip is the first FDA-cleared transcatheter mitral valve repair system and Mack said the device is used for function MR in about 65 percent of its is implants, and about 22 percent for degenerative MR cases. He said several new mitral repair devices are now entering clinical trials.
“MitraClip results are so good, it will be difficult for a new technology to replace it, let alone match it,” Mack said. “Most of these patients go home the next day.”
New repair technologies including the Harpoon and Neochord systems to replace damaged chordae tendineae. Both use Gortex chords that are anchored to the apex of the left ventricle using an epicardial anchor.
Transcatheter annuloplasty systems are being developed. Valtech Cardio Ltd. received CE mark for the Cardioband transcatheter annuloplasty system in September. A U.S. IDE study is planned for submission in 2016 and the first human procedures using the Cardioband system, modified for the treatment of tricuspid valve disease, are anticipated in late 2016. Read the article “Transcatheter Annuloplasty For Repair Versus Replacement in Functional Mitral Regurgitation.”
Mitralign and Cardiac Dimensions Carillon transcatheter annuloplasty systems are also in ongoing trials. Mitralign said its device is on track to received CE mark by the end of 2015. The Carillon already has CE mark and began enrollment for its FDA IDE trial in March, with and expected completion date in 2017.
Mack noted that if the results of the ongoing COAPT trial are successful, transcatheter mitral valve repair will explode. The study will examine the safety and effectiveness of the MitraClip to treat heart failure patients who have functional MR who are not appropriate for mitral value surgery. The trial results are expected by 2017.
Watch the video “Transcatheter Mitral Valve Repair Technologies.” An interview with Ted Feldman, M.D., FACC, MSCAI, FESC, cardiac cath lab director, Evanston Hospital, North Shore Health System, and principle investigator, Everest II MitraClip U.S. pivotal trial, at the Transcatheter Valve Therapies 2015 meeting.
Entry of Transcatheter Tricuspid Valve Interventions
The tricuspid valve (TV) was discussed at another new frontier at TCT. The TV is difficult to access due to its anatomic location, so tricuspid valve surgeries only account for about 5,000 procedures a year, as compared to about 50,000 mitral valve surgeries, said Jason Rogers, M.D., director of cardiovascular research at U.C. Davis Medical Center, Sacramento, Calif. Of these procedures, most are annuloplasty ring repairs, as replacement of the valve has a high rate of mortality. Rogers also pointed out that current TV measurements are designed to help surgeons, not interventionalists, so new standards need to be created for transcatheter repair and replacement procedures
“Currently our assessment of the tricuspid valve is very limited,” said Rebecca Hahn, M.D., FASE, director of invasive and valvular echocardiography, Columbia University Medical Center/New York-Presbyterian Hospital. She said new TV quantification standards via echo need to be developed. She explained TV insufficiency is an independent predictor of a worsening NYHA heart failure classification. However, there are many questions that need to be answered as transcatheter TV options are developed. These include when to intervene, if it should be at the time of a mitral valve surgery or before the right ventricle fails. Also with new devices, what is the best way to intervene, whether it is via annuloplasty rings, transcatheter repair or replacement.
“It is really an unmet need,” said Joachim Schofer, M.D., director of the Hamburg University Cardiovascular Center, Germany. “My desk is full of patients who need a tricuspid valve procedure who are in end-stage heart failure. Because valve replacement is not really an option for the tricuspid valve, I think there is a lot of opportunity for transcatheter replacement.” Schofer performed the first direct transcatheter tricuspid repair using the Mitralign annuloplasty system in March.
Several novel TV technologies were presented at TCT. Edwards Lifesciences also announced details of the 13 first-in-human compassionate use cases with its Forma transcatheter tricuspid repair system. All the patients had severe functional tricuspid regurgitation (TR) and were determined to be high-risk and deemed unsuitable for surgery by a heart team. All had clinical signs of heart failure. Twelve of the 13 patients had successful implantation of the Forma system. There were no deaths and no major clinical complications reported.
In August, Mitralign Inc. was granted an FDA IDE early feasibility study to examine its Percutaneous Tricuspid Valve Annuloplasty System (PTVAS). The SCOUT Study will take place in select centers in the United States, with Hahn serving as its principal investigator.
Advancements in TAVR and TMVR Technologies at TCT 2016
Watch the video VIDEO “Transcatheter Valve Technology Advancements at TCT 2016.” This is an interview Torsten Vahl, M.D., about advancements in transcatheter valve repair technology, including new devices for the aortic, mitral and tricuspid valves. Vahl is director of experimental and translational research and assistant professor of medicine, Columbia University Medical Center, Center for Interventional Vascular Therapy.
Watch the video “VIDEO: Transcatheter Mitral Valve Technology, Anatomical Challenges.” A discussion with Juan Granada, M.D., about transcatheter mitral valve advancements and device challenges at the Transcatheter Cardiovascular Therapeutics (TCT) 2016 annual meeting. Granada is executive director and chief scientific officer of the Cardiovascular Research Foundation's Skirball Center for Innovation.




 December 24, 2025
December 24, 2025 









