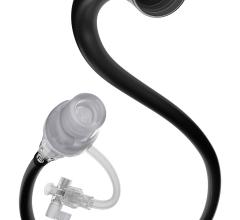
Medtronics Export XT aspiration catheter features a soft tip material with a short, beveled design for minimal vessel trauma and improved debris capture, plus a hydrophilic coating for greater lubricity.
Blood clots can occur almost anywhere in the body with potentially devastating implications. Typically, the first line of treatment for blood clots is drug therapy. Thrombolytic medications, such as tissue plasminogen activator (tPA), are approved for the immediate treatment of a clot in the brain (stroke) and for heart attack caused by a clot in a blood vessel of the heart. The best odds for survival exist when the drug is administered within 12 hours — but ideally within the first 90 minutes — after the heart attack starts, according to the AHA. For stroke patients, it’s recommended that thrombolytic medication be given within three hours of the onset of symptoms to help reduce the risk of damage and disability. In emergencies such as these, the thrombolytic medications are administered intravenously.
For patients outside the three-hour optimum window — estimated as the majority of stroke victims — catheter-based treatments offer a promising alternative. Catheters inserted into the arterial system, most commonly through the groin, deliver the clot-busting medication or removal device directly to the blockage.
The Society for Cardiovascular Angiography and Interventions (SCAI) is encouraging use of catheter-based treatments for stroke intervention, and last year launched a simulation-based course, “SCAI Core Curriculum in Introductory Neuro Rescue.” During the simulation sessions, cardiologists were trained on how to use a catheter-based clot-retrieval device to remove a clot and restore blood flow to the brain.
“Interventionalists are able to transfer their experience in catheter-based treatments from the heart to the brain, and together with neurologists and other specialists, we have the opportunity to dramatically impact the effects of stroke,” said Ziyad M. Hijazi, M.D., MPH, FSCAI, president of SCAI.
Devices such as Concentric Medical’s Merci Retriever provide physicians with an interventional option for stroke treatment that traps the clot and safely withdraws it.
Available in multiple configurations and sizes to match patient anatomy, the Merci Retriever is made with a flexible, shaped nitinol wire that allows delivery of the retriever in linear form using standard catheterization techniques. The distal end of the V series of Merci Retrievers is tightly coiled, which resists stretching and assists in dislodging clots. The proximal end is more loosely coiled, which facilitates wrapping and holding a dislodged clot, and filaments provide an additional mechanism for securing the clot during retrieval.
PCI Pitfall
Increasingly, percutaneous coronary interventions in the cath lab are replacing more invasive surgical procedures. These maneuvers, however, carry certain risks of their own. During a PCI, debris can be dislodged and travel into the bloodstream increasing the chance of a blockage or clot.
Aspiration catheters designed for vascular interventional procedures have proven successful in removing these blockages. And for STEMI patients, adding thrombus aspiration to primary percutaneous coronary intervention (PPCI) may further improve patient outcomes.
Published in the January issue of the Journal of the American College of Cardiology (Vol. 53, pages 309-315), the EXPIRA (Thrombectomy With Export Catheter in Infarct-Related Artery During Primary Percutaneous Coronary Intervention) prospective, randomized trial found that “thrombectomy prevents thrombus embolization and preserves microvascular integrity reducing infarct size, and it therefore represents a useful adjunctive therapy in PPCI.”
The device used in the study was Medtronic’s Export XT aspiration catheter. It features a soft tip material with a short, beveled design for minimal vessel trauma and improved debris capture, plus a hydrophilic coating. The “full-wall variable braiding” is designed to help eliminate segment joints and weak points in the catheter to improve kink resistance. It also allows enhanced deliverability because of the reportedly seamless transition of shaft stiffness from one end of the catheter to the other.
“Unlocking” the Clot
Ultrasound-accelerated thrombolysis represents an alternative to traditional catheter-directed thrombolysis that is said to dramatically reduce the amount of clot-dissolving drugs needed to break up blood clots.
“Ultrasound-accelerated thrombolysis involves the delivery of a pharmacological lytic agent (such as tPA) via an infusion catheter, with concurrent use of ultrasound energy delivered via the same catheter system,” said Michael C. Stoner, M.D., RVT, FACS, assistant professor of cardiovascular sciences, section of vascular and endovascular surgery, The Brody School of Medicine, East Carolina University, Greenville, NC.
The ultrasound and drug are administered simultaneously because it has been shown that ultrasound energy induces a temporary change in the structure of the clot that allows the drug to penetrate more efficiently into the inner reaches of the blockage.
“The ultrasound energy breaks up or softens the thrombus, and allows for better drug penetration and delivery,” explained Dr. Stoner.
When compared to catheter-directed thrombolysis, ultrasound-accelerated thrombolysis offers two potential advantages, he says. With improved drug delivery, the total dose-time product is reduced, which translates to 1: either a reduced lytic time (if using a standard dose of drug), or 2: a reduced drug dosage rate (if used over a standard time). Dr. Stoner and his colleagues use the technology as a mechanism to reduce drug delivery rate and improve thrombolysis safety. “So far, we’ve seen very few bleeding complications with use of this system, and no impact on efficacy,” Dr. Stoner said.
The system is the EKOS EndoWave catheter from EKOS Corp, which Dr. Stoners says his facility has been using for about two years.
“After a very quick learning curve, we have been using it almost exclusively for our thrombolysis cases. We heard about the product before its FDA approval, and were anxious to try it as a mechanism to improve the safety of thrombolysis.” Dr. Stoner said.
The EKOS EndoWave catheter system is a catheter-based drug delivery system utilizing high-frequency, low-power ultrasound. While treatment of DVT — a clot that forms in the deep vein in either the leg or pelvic region — is a logical indication for ultrasound-accelerated thrombolysis, says Dr. Stoner, the system can be used for native arterial thrombosis and bypass graft thrombosis. It could also be used with a smaller French-size system for intracranial/stroke applications.
For peripheral vessels, the EKOS Lysus system consists of 5.2 Fr. catheter with a central lumen and three separate lumens for local infusion of a thrombolytic drug through small pores in the catheter. An ultrasound core wire, located in the central lumen, contains multiple miniature ultrasound transducers 1 cm apart that delivers pulsed-wave ultrasound energy along the length of the catheter.
For More Information
www.medtronic.com
www.ekoscorp.com
www.concentric-medical.com




 January 19, 2026
January 19, 2026