
October 18, 2015 — Two-year results from the IN.PACT SFA study found the use of a drug-coated balloon was superior to conventional percutaneous transluminal angioplasty (PTA) in treating patients with superficial femoral artery (SFA) disease.
Findings were reported at the 27th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium. The study was also published in the Journal of the American College of Cardiology.
SFA disease remains a challenge to manage with no evidence-based standard treatment defined. Traditional PTA is associated with a high incidence of restenosis when used for anything but focal, noncomplex lesions and concerns persist about in-stent restenosis and stent fractures. Early study results with drug-coated balloons in randomized trials have been promising, but longer term results are lacking. The IN.PACT SFA study sought to assess the safety and efficacy of a drug-coated balloon versus standard PTA for the treatment of superficial femoral and proximal popliteal artery disease due to claudication and rest pain.
The prospective, multicenter, single-blinded trial enrolled 331 patients in a 2:1 randomization to a drug-coated balloon group (DCB, N=220) or standard PTA group (N=111). The primary efficacy endpoint was primary patency, defined as freedom from clinically-driven target lesion revascularization (CD-TLR) and duplex-derived restenosis. The primary composite safety endpoint was freedom from device and procedure-related death through 30 days, and freedom from target limb major amputation and clinically-driven TVR through two years.
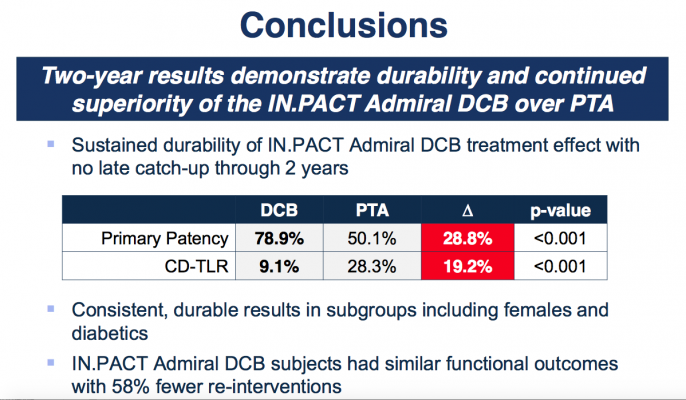
At two years, patients treated with DCB showed higher primary patency compared to PTA (78.9 percent vs. 50.1 percent, P<0.001). Freedom from CD-TLR was 91 percent for DCB compared to 72.2 percent for PTA (P<0.001). Results of the two-year primary safety composite was also positive for the DCB group (87.4 percent vs. 69.8 percent, p<0.001). There were no device or procedure-related deaths and no major amputations in either group through two-year follow-up, although mortality unrelated to the device/procedure was higher with DCB compared with PTA alone. Favorable DCB results were consistent and durable in female and diabetic subgroups. In addition, DCB patients achieved similar functional outcomes with 58 percent fewer re-interventions.
“The two-year data from IN.PACT SFA demonstrates the continued superiority of drug-coated balloons for treating patients with superficial femoral artery disease compared to percutaneous transluminal angioplasty,” said lead investigator John R. Laird Jr., M.D., professor of medicine and medical director of the Vascular Center at UC Davis Medical Center.
“With a sustained durability and no late catch-up after two years, this has the potential to drive a paradigm shift in how we treat SFA disease.”
The study was funded by Medtronic. Laird reported grant/research support from Medtronic and W.L. Gore; consulting fees/honoraria from Abbott Vascular, Bard Peripheral Vascular, Boston Scientific, Cordis and Medtronic; and major stock shareholder/equity in Syntervention, AngioSlide, BioCardia, Endoluminal Sciences, Reflow Medical, Eximo, Shockwave Medical, Ostial and PQ Bypass.
For more information: www.tctconference.com


 October 31, 2025
October 31, 2025 









