January 14, 2014 — Edwards Lifesciences Corp. announced it received investigational device exemption (IDE) approval from the U.S. Food and Drug Administration (FDA) to initiate a single-arm, non-randomized
clinical trial of the Sapien 3 transcatheter aortic heart valve in the treatment of intermediate risk patients with severe symptomatic aortic stenosis.
The company also completed enrollment in its U.S. clinical trial studying the Sapien 3 valve in the treatment of high-risk or inoperable patients.
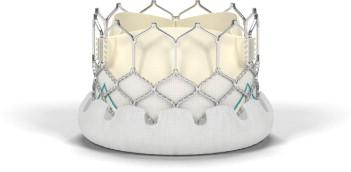
The Sapien 3 valve is the only transcatheter heart valve available to U.S. patients that can be delivered through a low-profile 14 French expandable sheath. It also has an outer skirt — a cuff of fabric surrounding the bottom of the frame — to provide a seal to address paravalvular leak. The Sapien 3 valve can be implanted with the transfemoral approach through an incision in the leg, as well as alternative access approaches. It is an investigational device that is not available commercially in any country; Edwards anticipates CE mark approval in the near future.
The U.S. trial of the Sapien 3 valve will enroll up to 1,000 patients with a Society of Thoracic Surgeons score of 4 to 8 percent, which indicates the average predicted risk of operative mortality at 30 days. All enrolled patients can receive a Sapien 3 valve.
"Last year, we completed enrollment in the first U.S. randomized controlled trial involving intermediate risk patients with severe aortic stenosis,” said Larry Wood, corporate vice president, transcatheter heart valves, Edwards Lifesciences. “This unique dataset of 2,000 patients receiving surgery or transcatheter valve replacement will provide a thorough baseline comparison for this new study of the Sapien 3 valve in intermediate risk patients."
For more information: www.edwards.com